* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download MS Science - Fair Lawn Public Schools
Low-Income Home Energy Assistance Program wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Regenerative brake wikipedia , lookup
Low-carbon economy wikipedia , lookup
World energy consumption wikipedia , lookup
Energy storage wikipedia , lookup
Compressed air energy storage wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
International Energy Agency wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Alternative energy wikipedia , lookup
Negawatt power wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Micro combined heat and power wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Energy harvesting wikipedia , lookup
Internal energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
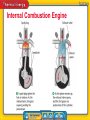
Chapter Introduction Lesson 1 Thermal Energy, Temperature, and Heat Lesson 2 Thermal Energy Transfers Lesson 3 Using Thermal Energy Chapter Wrap-Up How can thermal energy be used? What do you think? Before you begin, decide if you agree or disagree with each of these statements. As you view this presentation, see if you change your mind about any of the statements. Do you agree or disagree? 1. Temperature is the same as thermal energy. 2. Heat is the movement of thermal energy from a hotter object to a cooler object. 3. It takes a large amount of energy to significantly change the temperature of an object with a low specific heat. Do you agree or disagree? 4. The thermal energy of an object can never be increased or decreased. 5. Car engines create energy. 6. Refrigerators cool food by moving thermal energy from inside the refrigerator to the outside. Thermal Energy, Temperature, and Heat • How are temperature and kinetic energy related? • How do heat and thermal energy differ? Thermal Energy, Temperature, and Heat • thermal energy • temperature • heat Kinetic and Potential Energy • Potential energy is stored energy due to the interaction between two objects. • The potential energy plus the kinetic energy of an object is the mechanical energy of the object. What is thermal energy? • Every solid, liquid, and gas is made up of trillions of tiny particles that are constantly moving. • Because particles are in motion, they have kinetic energy. • The faster particles move, the more kinetic energy they have. What is thermal energy? (cont.) • The greater the average distance between particles, the greater the potential energy of the particles. • Thermal energy is the sum of the kinetic energy and the potential energy of the particles that make up a material. The potential energy of the soccer ball depends on the distance between the ball and Earth. The potential energy of the particles of matter depends on their distance from one another. What is thermal energy? (cont.) • Mechanical energy describes the energy of one object. • Thermal energy describes the energy of the particles that make up a solid, liquid, or gas. What is temperature? • Scientists define temperature in terms of kinetic energy. • Temperature represents the average kinetic energy of the particles that make up a material. What is temperature? (cont.) temperature from Latin temperatura, means “moderating, tempering” What is temperature? (cont.) • The greater the average kinetic energy of particles, the greater the temperature. • The particles in warmer air move at a greater average speed than the particles in colder air. What is temperature? (cont.) How are temperature and kinetic energy related? What is temperature? (cont.) • Temperature and thermal energy are related, but they are not the same. • The particles that make up liquid and solid water have different potential energies and, therefore, different thermal energies. What is temperature? (cont.) • Thermometers are used to measure temperature. • Common temperature scales are Celsius (°C), Kelvin (K), and Fahrenheit (°F). What is heat? • The movement of thermal energy from a warmer object to a cooler object is called heat. • All objects have thermal energy; however, you heat something when thermal energy transfers from one object to another. • The rate at which heating occurs depends on the difference in temperatures between the objects. What is heat? (cont.) How do heat and thermal energy differ? • The greater the distance between two particles or two objects, the greater the potential energy. • Heat is the movement of thermal energy from a warmer object to a cooler object. • When thermal energy moves between a material and its environment, the material’s temperature changes. Because particles are in motion, what type of energy do they have? A. thermal energy B. potential energy C. mechanical energy D. kinetic energy Particles that make up liquid and solid water have different potential energies, and therefore also have which of the following? A. different thermal energies B. different temperatures C. different kinetic energies D. the same thermal energy Which term refers to the average kinetic energy of the particles that make up a material? A. heat B. temperature C. potential energy D. thermal energy Do you agree or disagree? 1. Temperature is the same as thermal energy. 2. Heat is the movement of thermal energy from a hotter object to a cooler object. Thermal Energy Transfers • What is the effect of having a small specific heat? • What happens to a material when it is heated? • In what ways can thermal energy be transferred? Thermal Energy Transfers • radiation • conduction • thermal conductor • thermal insulator • specific heat • thermal expansion • thermal contraction • convection • convection current How is thermal energy transferred? • Thermal energy is transferred in three ways. • by radiation • by conduction • by convection Radiation • The transfer of thermal energy from one material to another by electromagnetic waves is called radiation. • Radiation is the only way thermal energy can travel from the Sun to Earth, because space is a vacuum. • Radiation also transfers thermal energy through solids, liquids, and gases. Radiation (cont.) vacuum Science Use a space that contains little or no matter Common Use a device for cleaning carpets and rugs that uses suction Conduction • When particles with different kinetic energies collide, the particles with higher kinetic energy transfer energy to particles with lower kinetic energy. • The transfer of thermal energy between materials by the collisions of particles is called conduction. • Conduction continues until the thermal energy of all particles in contact is equal. Conduction (cont.) • The hot air transfers thermal energy to, or heats, the cool lemonade by conduction. • Eventually the kinetic thermal energy and temperature of the air and the lemonade will be equal. Conduction (cont.) • A thermal conductor is a material through which thermal energy flows easily. • A thermal insulator is a material through which thermal energy does not flow easily. Conduction (cont.) • The amount of thermal energy required to increase the temperature of 1 kg of a material by 1°C is called specific heat. • Water’s high specific heat helps prevent your body from overheating. • Changing the temperature of a material with a low specific heat is easier than to change the temperature of a material with a high specific heat. In a hot car, the temperature of thermal conductors, such as the safety-belt buckles, increases more quickly than the temperature of thermal insulators, such as the seat material. Conduction (cont.) What does it mean if a material has a low specific heat? Thermal Expansion and Contraction • Thermal contraction is a decrease in a material’s volume when its temperature decreases. • Thermal expansion is an increase in a material’s volume when its temperature increases. • Thermal expansion and contraction are most noticeable in gases, less noticeable in liquids, and the least noticeable in solids. Thermal Expansion and Contraction (cont.) What happens to the volume of a gas when it is heated? Convection • Convection is the transfer of thermal energy by the movement of particles from one part of a material to another. • Convection only occurs in fluids. convection from Greek convectionem, means “the act of carrying” This cycle of cooler water sinking and forcing warmer water upward is an example of convection. Convection (cont.) What are the three processes that transfer thermal energy? Convection (cont.) • The movement of fluids in a cycle because of convection is a convection current. • Convection currents circulate the water in Earth’s oceans and other bodies of water. Convection Currents in Earth’s Atmosphere • When a material has a low specific heat, transferring a small amount of energy to the material increases its temperature significantly. • Thermal energy can be transferred through radiation, conduction, or convection. • When a material is heated, the thermal energy of the material increases and the material expands. Which term refers to a material through which thermal energy flows easily? A. convection current B. specific heat C. thermal conductor D. thermal insulator Which describes an increase in a material’s volume when its temperature increases? A. conduction B. thermal expansion C. thermal conductor D. thermal contraction What term describes the transfer of thermal energy by the movement of particles from one part of a material to another? A. convection B. conduction C. thermal contraction D. thermal expansion Do you agree or disagree? 3. It takes a large amount of energy to significantly change the temperature of an object with a low specific heat. 4. The thermal energy of an object can never be increased or decreased. Using Thermal Energy • How does a thermostat work? • How does a refrigerator keep food cold? • What are the energy transformations in a car engine? Using Thermal Energy • heating appliance • thermostat • refrigerator • heat engine Thermal Energy Transformations • Thermostats transform thermal energy into mechanical energy that switch heaters on and off. • Even though many devices transform energy from one form to another or transfer energy from one place to another, the total amount of energy does not change. Heating Appliances • A device that converts electric energy into thermal energy is a heating appliance. • Curling irons, coffeemakers, and clothes irons are some examples of heating appliances. Thermostats A thermostat is a device that regulates the temperature of a system. thermostat from Greek therme, meaning “heat”; and statos, meaning “a standing” Thermostats (cont.) • Most thermostats contain a bimetallic coil made of two types of metal joined together and bent into a coil. • The metal on the inside of the coil expands and contracts more than the metal on the outside of the coil. • When a room warms or cools, the thermal energy causes the bimetallic coil to uncurl slightly or tighten, which turns the furnace off or on. Thermostats (cont.) How does the bimetallic coil in a thermostat respond to heating and cooling? Refrigerators • A device that uses electric energy to transfer thermal energy from a cooler location to a warmer location is called a refrigerator. • In a refrigerator, a coolant is pumped through pipes on the inside and the outside of the refrigerator. Coolant in a refrigerator moves thermal energy from inside to outside the refrigerator. The coolant, which begins as a liquid, passes through an expansion valve and cools. As the cold gas flows through pipes inside the refrigerator, it absorbs thermal energy from the refrigerator compartment and vaporizes. Refrigerators (cont.) How does a refrigerator keep food cold? Heat Engines • A heat engine is a machine that converts thermal energy into mechanical energy. • When a heat engine converts thermal energy into mechanical energy, the mechanical energy moves the vehicle. • Most cars, buses, boats, trucks, and lawn mowers use a type of heat engine called an internal combustion engine. Internal Combustion Engine Internal Combustion Engine Heat Engines (cont.) What is one form of energy that is output from a heat engine? • A bimetallic coil inside a thermostat controls a switch that turns a heating or cooling device on or off. • A refrigerator keeps food cold by moving thermal energy from the inside of the refrigerator out to the refrigerator’s surroundings. • In a car engine, chemical energy in fuel is transformed into thermal energy. Some of this thermal energy is then transformed into mechanical energy. Curling irons, coffeemakers, and clothes irons are some examples of what? A. thermostats B. refrigerators C. heat engines D. heating appliances Which is pumped through pipes on the inside and the outside of a refrigerator? A. water B. ice C. coolant D. bimetallic coil Which term refers to a device that regulates the temperature of a system? A. heat engine B. heating appliance C. refrigerator D. thermostat Do you agree or disagree? 5. Car engines create energy. 6. Refrigerators cool food by moving thermal energy from inside the refrigerator to the outside. Key Concept Summary Interactive Concept Map Chapter Review Standardized Test Practice Thermal energy can be transferred by conduction, radiation, and convection. Thermal energy also can be transformed into other forms of energy and used in devices such as thermostats, refrigerators, and automobile engines. Lesson 1: Thermal Energy, Temperature, and Heat • The temperature of a material is the average kinetic energy of the particles that make up the material. • Heat is the movement of thermal energy from a material or area with a higher temperature to a material or area with a lower temperature. • When a material is heated, the material’s temperature changes. Lesson 2: Thermal Energy Transfers • When a material has a low specific heat, transferring a small amount of energy to the material increases its temperature significantly. • When a material is heated, the thermal energy of the material increases and the material expands. • Thermal energy can be transferred by conduction, radiation, or convection. Lesson 3: Using Thermal Energy • The two different metals in a bimetallic coil inside a thermostat expand and contract at different rates. The bimetallic coil curs and uncurls, depending on the thermal energy of the air, pushing a switch that turns a heating or cooling device on or off. • A refrigerator keeps food cold by moving thermal energy from inside the refrigerator out to the refrigerator’s surroundings. • In a car engine, chemical energy in fuel is transformed into thermal energy. Some of this thermal energy is then transformed into mechanical energy. Which describes the sum of the kinetic energy and the potential energy of the particles that make up a material? A. heat B. temperature C. thermal energy D. mechanical energy The transfer of thermal energy from one material to another by electromagnetic waves is called what? A. conduction B. radiation C. specific heat D. thermal expansion Which refers to a decrease in a material’s volume when its temperature decreases? A. conduction B. radiation C. thermal contraction D. thermal expansion Which describes the amount of thermal energy required to increase the temperature of 1 kg of a material by 1°C? A. thermal expansion B. specific heat C. convection D. conduction What term refers to the part of a thermostat that expands and contracts to turn a furnace on and off? A. coolant B. piston C. bimetallic coil D. heat engine What term refers to the movement of thermal energy from a warmer object to a cooler object? A. temperature B. heat C. potential energy D. kinetic energy Which term refers to a material through which thermal energy does not flow easily? A. convection current B. thermal contraction C. thermal conductor D. thermal insulator Which is the movement of fluids in a cycle because of convection? A. convection current B. thermal contraction C. thermal expansion D. thermal conductor Which term describes the transfer of thermal energy between materials by the collisions of particles? A. convection B. conduction C. thermal expansion D. thermal contraction Which describes a machine that converts thermal energy into mechanical energy? A. piston B. thermostat C. heat engine D. heating appliance