* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Neuroanatomy wikipedia , lookup
Central pattern generator wikipedia , lookup
Metastability in the brain wikipedia , lookup
Biological neuron model wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neural coding wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Development of the nervous system wikipedia , lookup
Optogenetics wikipedia , lookup
Nervous system network models wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Single-unit recording wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Transcript
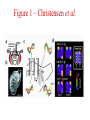
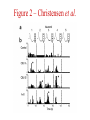
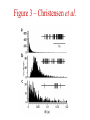
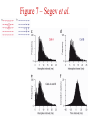
Multi-Electrode Arrays (MEAs) March 25, 2005 Introduction Multi-electrode Arrays, or MEAs, are quickly becoming a common tool to investigate patterns of activity. Often see 4-, 8-, 16-, or even 60-channel array – experimenter’s choice Made of : microwires (stainless steel, tungsten, platinum/iridium) coated with nonconductive polymers (Teflon or formvar), or silicon; tip diameter of 1-5 mm Horizontal array: inter-electrode separation of 400-500 mm Vertical array: interelectrode separation 250-300 mm Luo & Katz, 2003 recording in a behaving mouse Response of a neuron from a CBA male during investigation of anesthetized stimulus animals including BALBc male, CBA male, BALBc female, and CBA female, demonstrating excitation by a BALBc male and inhibition by a CBA female. Sound, playback of spiking activity. Colored bar on the right, the cell’s mean firing rate; red, excitation; blue, inhibition. Note the gradual increase of neuronal activity, strong excitatory response to its investigation of the face area of the BALBc male, and the inhibition following strong excitation. An economical multi-channel cortical electrode array for extended periods of recording during behavior Rennaker, R.L., Ruyle, A.M., Street, S.E. and A.M. Sloan. (2005) J. Neurosci Meth 142:97-105. Figure 1 – Rennaker et al. Figure 2 – Rennaker et al. Figure 3 – Rennaker et al. Figure 4 – Rennaker et al. Figure 5 – Rennaker et al. Figure 6 – Rennaker et al. Figure 7 – Rennaker et al. Multi-unit recordings reveal contextdependent modulation of synchrony in odor-specific neural ensembles Christensen, T.A., Pawlowski, V.M., Lei, H. and J.G. Hildebrand (2000) Nat Neurosci 3:927-931. Christensen et al. - Introduction • Studies in Manduca Sexta, a moth, that is a common model for insect olfaction. • The antennal lobe (AL) is a structural and functional analogue of the mammalian olfactory bulb (OB) • Glomerulus = functional unit of the AL (and OB) that receives odor information from a single type of sensory neuron • each glomerulus then sends odor-specific information out to higher level brain centers via the projection neurons (PNs) • glomeruli are thought to be activated in stereotypical patterns that then make up an “odor map” of each odor Figure 1 – Christensen et al. Figure 2 – Christensen et al. Figure 3 – Christensen et al. Figure 4 – Christensen et al. Figure 5 – Christensen et al. Recording spikes from a large fraction of the ganglion cells in a retinal patch Segev, R., Goodhouse, J., Puchalla, J. and M.J. Berry II 2003 Nature Neurosci 7:1155-1162. Segev et al. - Introduction • Why the retina? Promising for systematic study of large populations of neurons because of its modular organization – recording from a small patch of ganglion cells should sample its full functional diversity • Limitations of multielectrode arrays? Sorting the signals obtained into spike trains from individual neurons • This paper: Development of a new method of recording and spike sorting that uses a dense array and combines signals from up to 30 electrodes to sort spikes. Records from ganglion cells, output cells of retina. Segev et al. - Introduction How accomplished? 1. Find the average voltage pattern on the array when a ganglion cell fires a spike 2. Use an iterative algorithm to match multiple spike patterns to the raw data Because every ganglion cell occupies a unique position in space, and because extracellular signals decay rapidly with distance, each ganglion cell produces a unique pattern of activity on the dense array This unique pattern can be used to identify the source of overlapping spikes, which might appear ambiguous if using only one electrode. Figure 1 – Segev et al. Figure 2 – Segev et al. Figure 3 – Segev et al. Figure 3 – Segev et al. Figure 3 – Segev et al. Figure 4 – Segev et al. Figure 4 – Segev et al. Figure 5 – Segev et al. Figure 6 – Segev et al. Figure 7 – Segev et al. Figure 7 – Segev et al. Figure 8 – Segev et al.