* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Confocal Microscopy
Optical amplifier wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Night vision device wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Retroreflector wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Gaseous detection device wikipedia , lookup
Optical aberration wikipedia , lookup
Chemical imaging wikipedia , lookup
Upconverting nanoparticles wikipedia , lookup
Optical coherence tomography wikipedia , lookup
3D optical data storage wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Fluorescence correlation spectroscopy wikipedia , lookup
Fluorescence wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
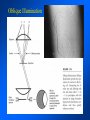
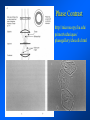
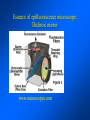
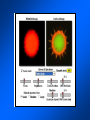
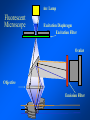
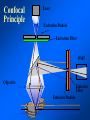
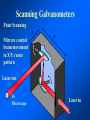
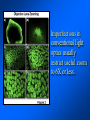
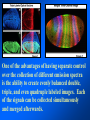
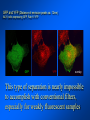
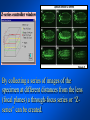
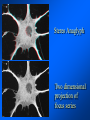
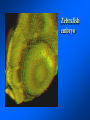
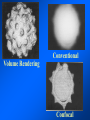
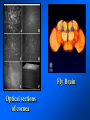
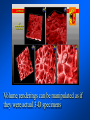
Brightfield microscopy • Generally only useful for stained biological specimens • Unstained cells are virtually invisible Oblique Illumination Phase Contrast http://microscopy.fsu.edu/ primer/techniques/ phasegallery/chocells.html Aberrations • Spherical aberration – Most severe – Immersion fluid • Field curvature • Chromatic aberration • Astigmatism, comma • http://micro.magnet.fsu.edu/primer/lightandcolor/ opticalaberrations.html Phenomenon of fluorescence Probes.invitrogen.com Jablonski diagram: Absorption of photon elevates fluorophore to excited singlet state S1’ Nonradiative decay to lowest energy singlet excited state S1 Decay to ground state by emission of a photon Non-radioactive decay to triplet state leads to photobleaching Molecular Expressions website Ideal fluorophore characteristics • High quantum efficiency • Slow photobleaching • For live cells: excitation wavelengths nonphototoxic to cells • Little overlap with autofluorescence – Mammalian cells: Flavoprotein, pigment – Plant cells: chlorophyll Fluorescent proteins: GFP Tsien Lab (UCSD) How we observe fluorescence • Black light – Not enough sensitivity • Filters – Bleed-through • Darkfield fluorescence microscopy Epifluorescence microscopy Nikon Microscopy U Essence of epifluorescence microscope: Dichroic mirror www.microscopyu.com Examples of Fluorescence Confocal Microscopy The term “confocal” means “having the same focus” This is accomplished by focusing the condensor lens to the same focal plane as the objective lens. • • • • • • • Reduced blurring of the image from light scattering Increased effective resolution Improved signal to noise ratio Clear examination of thick specimens Z-axis scanning Depth perception in Z-sectioned images Magnification can be adjusted electronically Laser • Acronym: Light Amplification by Stimulated Emission of Radiation • Ordinary light emission: Comes from spontaneous decay of excited state to ground levels • Stimulated emission: molecule remains in excited state until stimulated to emit by incoming light that is insufficient to raise it to the next higher excited state Conventional Fluorescence Confocal The difference in resolution can be significant in those specimens which are too thick to fit entirely within the focal plane of the lens. Optical section of an aphid showing internal structure of an intact animal If this is coupled with a point source of illumination and a matching point source of detection Pinhole 1 Pinhole 2 Specimen Detector Condenser Lens Objective Lens Modified from: Handbook of Biological Confocal Microscopy. J.B.Pawley, Plennum Press, 1989 Arc Lamp Fluorescent Microscope Excitation Diaphragm Excitation Filter Ocular Objective Emission Filter Confocal Principle Laser Excitation Pinhole Excitation Filter PMT Objective Emission Filter Emission Pinhole In a confocal microscope only a relatively small portion of the specimen is illuminated at a time whereas in a conventional fluorescence microscope a much broader area is illuminated. If the source of illumination is truly a point and it is focused to a point then only a single point in the specimen will imaged at any one time. Either the specimen must be moved to create a complete view or the beam must be scanned in a raster pattern. One way to accomplish this is to pass the illumination through a series of pinholes that have been arranged in a pattern. As this disk is spun it will create a raster pattern and the light coming back through the pinholes will be confocal. The pinholes in a spinning disk system act as both the point sources and confocal apertures. Spinning disk confocals: 1) Can image in “real” time provided that the disk is spun quickly enough 2) Can use a variety of light sources 3) Can be retrofitted to many existing fluorescence microscopes Spinning disk confocals: 1) Are inefficient and require a very bright illumination and fluorescence 2) Cannot use sensitive light detectors such as photomultiplier tubes Scanning Galvanometers Point Scanning x Mirrors control beam movement in X/Y raster pattern y Laser out To Microscope Laser in Some scan mirror systems are able to be rotated which can result in a rotation of the raster pattern By creating an image in a point by point manner the confocal microscope functions as a point scanning/signal detecting device and like an SEM magnification can be increased by scanning a smaller portion of the specimen Imperfections in conventional light optics usually restrict useful zoom to 6X or less. A modern confocal system consists of the microscope, associated lasers, the scan head with detectors and confocal apertures and a computer system that controls the scanning, adjusts the illumination, collects the signal, displays the images and stores the data for later image processing and analysis. A laser scanning confocal microscope has many components including a way for several different lasers to provide excitation wavelengths and several separate detectors for various emission wavelengths Although confocal microscopy can be done in the reflected (light backscattered) or even transmitted mode most systems are optimized for fluorescence. Light Sources - Lasers • • • • • • • Argon UV ArUV Solid State Violet Argon Ar Krypton-Ar ArKr Helium-Cad HeCd Helium-Neon GreNe Helium-Neon HeNe 351-364 nm 405 nm 488-514 nm 488-568-648 nm 442 nm 543 nm 633 nm Excitation - Emission Peaks Fluorophore DAPI FITC Bodipy Tetra-M-Rho L-Rhodamine Texas Red CY5 EXPeak EM peak 358 496 503 554 572 592 649 461 518 511 576 590 610 666 % Max Excitation at 488 568 647 nm 0 87 58 10 5 3 1 0 0 1 61 92 45 11 0 0 1 0 0 1 98 Since the illumination wavelengths available are often limited the selection of matching fluorochromes is very important. In a conventional confocal scan head the photons returning from the specimen are separated based on their energies (color) by passing them through a series of filters and collecting each on separate PMTs One significant advance is how some systems separate the emission spectrum (signal) by wavelength and using slits sample those specific wavelengths using separate PMTs. One of the advantages of having separate control over the collection of different emission spectra is the ability to create evenly balanced double, triple, and even quadruple labeled images. Each of the signals can be collected simultaneously and merged afterwards. – Spectral properties of the available dyes limit the experimental freedom. – Often it is even difficult to clearly separate two fluorescence markers. – With more markers, the problem grows increasingly complex. Cross-talk between the FP variants at the excitation and emisson level Fluorescent Proteins are essential for life science studies. However, overlapping emission AND excitation spectra and corresponding crosstalk makes combinations difficult for imaging! (especially true for multiphoton imaging) Heavy overlap! GFP and YFP (Distance of emission peaks ca. 12nm) A431 cells expressing GFP, Rab11-YFP GFP YFP overlay This type of separation is nearly impossible to accomplish with conventional filters, especially for weakly fluorescent samples By collecting a series of images of the specimen at different distances from the lens (focal planes) a through-focus series or “Zseries” can be created. The ability to collect data in the X,Y, and Z dimensions enables one to create an image of the specimen as if it were be observed from an orthogonal plane. Stereo pair images can be created from a stack of confocal images by a technique known as “pixel shifting” In pixel shifting two separate 2-D projections of the data set are created by shifting adjacent image planes slightly out of registry creating a pseudo-left and pseudo-right projection. The two images can be colorize to produce an anaglyph stereo pair. (PC12 cell stained for microtubules) Stereo Anaglyph Two dimensional projection of focus series Pacific coral in backscattered light mode Zebrafish embryo Muscle cells 3D Image Reconstruction y z x By accessing information in all three dimensions a 3-D reconstruction of the data is possible 3D Image Reconstruction y y z y z x 3D Image Reconstruction y y z z x y Conventional Volume Rendering Confocal Fly Brain Optical sections of cornea Volume renderings can be manipulated as if they were actual 3-D specimens Applications • Probe Ratioing – Calcium Flux (Indo-1, Fluo-3) – pH indicators (BCECF, SNARF) Molecule-probe Calcium - Indo-1 Magnesium - Mag-Indo-1 Calcium-Fluo-3 Calcium - Fura-2 Calcium - Calcium Green Phospholipase A - Acyl Pyrene Excitation 351 nm 351 nm 488 nm 363 nm 488 nm Emission 405, >460 nm 405, >460 nm 525 nm >500 nm 515 nm 351 nm 405, >460 nm “Exotic” Applications • Release of “Caged” compounds • Fluorescence Recovery After Photobleaching (FRAP) (UV line) • Fluorescence Resonance Energy Transfer (FRET) “Caged” Photoactivatable Probes Nitrophenyl blocking groups e.g. nitrophenyl ethyl ester undergoes photolysis upon exposure to UV light at 340-350 nm Examples • • • • • • • Glutamate Norepinephrine IP3 cAMP cGMP ATP Ca++ Applications • Organelle Structure • & Function – – – – Mitochondria (Rhodamine 123) Golgi (C6-NBD-Ceramide) Actin (NBD-Phalloidin) Lipid (DPH)