* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Presentation
Survey
Document related concepts
Transcript
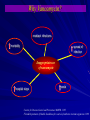
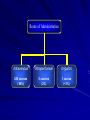
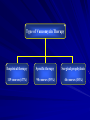
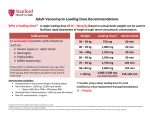
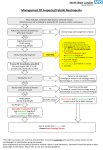
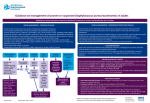
Drug Utilization Evaluation of Vancomycin at King Khalid University Hospital By: Dina I. Fouda, M.Sc. Pharm. candidate Supervised by: Dr. Lamya AL Naim Dr. Iman Zaghloul Dr. Ahmed Albarraq Major adviser Faculty Co-advisor External Co-adviser Outline Introduction to Vancomycin Why Vancomycin? CDC Guidelines TDM Objectives Methodology Results & Discussion Limitations Recommendations Conclusion Introduction Vancomycin is a glycopeptide antibiotic with bactericidal activity against gram - positive microorganisms. Approved by FDA in 1958. Johnson S.V., et al. Pharmacotherapy. 1995 Introduction In the past, it was used as a 2nd or 3rd line agent due to availability of: • Equally efficacious • Less toxic alternatives • Less expensive Johnson S.V., et al. Pharmacotherapy. 1995 Why Vancomycin? Over the last 15–20 years, the utilization of Vancomycin has increased because of the dramatic increase in MRSA, MRSE and Ampicillin resistant enterococci. MRSA = methicillin-resistant Staphylococcus aureus MRSE = methicillin-resistant Staphylococcus epidermidis Johnson S.V., et al. Pharmacotherapy. 1995 Percentage of Nosocomial Enterococcal Infections in United States 7.9% 0.3% 1988 1989 1990 1991 1992 1993 Year CDC. Nosocomial enterococci resistant to vancomycin -- United States, 1989-1993. MMWR 1993 Why Vancomycin? Problems: The lack of available antimicrobial therapy for VRE infections , because most VRE are also resistant to drugs previously used to treat such infections (e.g., aminoglycosides and ampicillin). VRE therapy requires expensive & not readily available antibiotics (e.g Linezolid & Teicoplanin). The possibility that the vancomycin-resistant genes present in VRE can be transferred to other gram-positive microorganisms (e.g., Staphylococcus aureus). Vancomycin has an important role in serious infections and we do not want to loose its efficacy. VRE=Vancomycin-resistant enterococci Centers for Disease Control and Prevention. MMWR . 1995 Why Vancomycin? resistant infections morbidity spread of infection Inappropriate use of vancomycin hospital stays costs Centers for Disease Control and Prevention. MMWR . 1995 Florida Department of Health. Guidelines for control of antibiotic resistant organisms. 1999 Why Vancomycin? In 1995, the Centers for Disease Control and Prevention (CDC) published guidelines for the use of vancomycin. Centers for Disease Control and Prevention. MMWR.. 1995 Guidelines published by the Centers for Disease Control and Prevention (CDC) Situations in which the use of vancomycin is appropriate or acceptable 1. 2. 3. 4. 5. For treatment of serious infections due to β-lactam resistant Grampositive microorganisms. For treatment of infections because of Gram positive microorganisms in patients with serious allergy to beta-lactam antimicrobials. Prophylaxis, as recommended by the American Heart Association, for bacterial endocarditis following certain procedures in high-risk patients. Prophylaxis for major surgical procedures involving implantation of prosthetic materials or devices. When antibiotic-associated colitis fails to respond to metronidazole therapy or is severe and potentially life-threatening. Centers for Disease Control and Prevention. MMWR.. 1995 Guidelines published by the Centers for Disease Control and Prevention (CDC) Situations in which the use of vancomycin should be discouraged 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Routine surgical prophylaxis. Treatment in response to a single blood culture positive for coagulase-negative staphylococcus, if other blood cultures taken during the same time frame are negative. Continued empiric use for presumed infections in patients whose cultures are negative for β-lactam–resistant gram-positive microorganisms. Empiric antimicrobial therapy for a febrile neutropenic patient. Systemic or local prophylaxis for infection or colonization of indwelling central or peripheral intravascular catheters. Selective decontamination of the digestive tract. Eradication of MRSA colonization. Primary treatment of antibiotic-associated colitis. Routine prophylaxis for very low-birth weight infants (i.e., infants who weigh<1,500 g). Routine prophylaxis for patients on continuous ambulatory peritoneal dialysis or hemodialysis. Treatment (chosen for dosing convenience) of infections caused by β-lactam– sensitive gram-positive microorganisms in patients who have renal failure. Use of vancomycin solution for topical application or irrigation. Centers for Disease Control and Prevention. MMWR . 1995 Guidelines published by National Comprehensive Cancer Network (NCCN) Indication consider appropriate at KKUH but NOT part of CDC guidelines 1. Empiric anti-microbial therapy for a febrile neutropenic patient, when there is strong evidence at the onset that the patient has an infection because of Gram-positive microorganisms. National Comprehensive Cancer Network. NCCN,. 2005 Why Vancomycin? Vancomycin considered a restricted antibiotic . Antimicrobial advisory committee at KKUH noticed a tremendous increase in vancomycin orders by non Infectious Disease specialties. KKUH = King Khalid University Hospital Vancomycin Therapeutic Drug Monitoring (TDM) The need for TDM in patients receiving Vancomycin is remaining controversial. Vancomycin demonstrates concentration-independent killing in its effect on bacteria. Measuring serum Vancomycin levels is to ensure an adequate trough serum level for efficacy rather than to predict or prevent toxicity. Vancomycin Therapeutic Drug Monitoring (TDM) One large problem, however, remains for vancomycin: the lack of standardized targets for serum concentration monitoring. The ideal vancomycin dosing regimen: Peak= 20 - 40 mg/L , Trough= 5 – 20 mg/L. Vancomycin Therapeutic Drug Monitoring (TDM) One large problem, however, remains for vancomycin: the lack of standardized targets for serum concentration monitoring. The ideal vancomycin dosing regimen: Peak= 20 - 40 mg/L , Trough= 5 – 20 mg/L. Vancomycin concentration sampling should be repeated if there is a change in renal function or lack of therapeutic response. Nephrotoxicity Vancomycin alone has a low potential for causing nephrotoxicity ( ≤5% ) in the absence of other factors that can adversely affect renal function. The nephrotoxicity of Vancomycin is increased to 43% when it is co-administered with nephrotoxic drugs. Cantú TG et al. Serum vancomycin concentrations:reappraisal of their clinical value. 1994 Evaluate the appropriateness of vancomycin use in KKUH in terms of Main objectives Indications in accordance with CDC guidelines Appropriate application Of TDM Secondary objectives Incidence of Nephrotoxicity Estimated acquisition cost Methodology Methodology Study Design and Population : • A retrospective review of medical charts & computerized databases of all patients who received a course of vancomycin between January and June 2005 during there admission to KKUH. KKUH = King Khalid University Hospital Methodology Data Collection: Data collection form was used to collect data. Divided into 6 categories: 1. 2. 3. 4. 5. 6. Demographic Data Hospital departments Appropriateness of therapy Dosing regimen Laboratory test Cost Methodology Statistical Analysis: • The data was coded & entered into SPSS version 14. • Descriptive statistics were used to evaluate most of the parameters. • Continuous variables were reported as a range. • Paired t-test was used to compare some of the parameters before and after the use of Vancomycin. SPSS= Statistical Package for Social Sciences S.D. = Standard Deviations Results & Discussion Six months period 222 patients 255 courses of vancomycin 176 adults 79 neonates & pediatric patients Age 18 – 95 Years Age < 1 month – 17 years Duration of course 1 – 74 days Route of Administration Intravenous Intraperitoneal Irrigation 249 courses ( 98%) 5 courses (2%) 1 course (<1%) Type of Vancomycin Therapy Empirical therapy Specific therapy Surgical prophylaxis 119 courses (47%) 90 courses (35%) 46 courses (18%) Appropriateness of Vancomycin therapy 60% 51% 49% 50% 40% 30% 20% 10% 0% Appropriate Inappropriate Appropriateness of Vancomycin therapy in different DUE studies 80% 70% 72% 72% 60% 54% 60% 49% 50% 40% 32% 33% 35% 40% Inappropriate 30% 20% 10% 0% Sin Ho Ho KS US US US Fra US ng ng A( ga A, A, A, A, nc po KK e, K K 19 19 19 2 00 on on re, 94 95 96 19 UH 1 g, g, 99 ), 19 2 2 00 00 99 20 0 2 05 Categories of inappropriate Vancomycin use Continued empiric use with culture -ve Routine surgical prophylaxis Treatment of bacteria sensitive to Blactam Gm +ve in non allergic patients 12% 5% 3% 2% 1%1% Routine empiric use for febrile neutropenic patient Treatment without evidence of a resistant Gm+ve infection 13% Single blood culture +ve to staphylococcus epidermidis 14% 49% Local prophylaxis for infection peripheral intravascular catheter Use of vancomycin solution for irrigation Eradication of MRSA colonization Compare with Hong Kong study Continued empiric use in patients whose culture negative KKUH 49% PWH 71% Appropriateness of Vancomycin use by different departments 60% 50% ICU 55% ICU 47% 40% 30% Medical 25% Surgical 28% Medical 23% Surgical 22% 20% 10% 0% Appropriate Inappropriate Appropriateness of Vancomycin use by the type of ICU CCU 37% 40% NICU 31% 35% 30% 25% 20% NICU 15% PICU 19% MICU 23% CCU PICU 20% 19% MICU 15% 15% SICU 6% 10% 5% 0% Appropriate Inappropriate SICU 15% Appropriateness of Vancomycin prescribing by medical service 9% 10% 13.7% 14% 12% 8% 6% Appropriate Inappropriate 4% 2% 0% y log ino cr do En gy olo ur Ne y og iol rd y Ca l og e ro as ph ise Ne sd ou cti fe In i ce y dic er rg me Su re ca s al ric iat itic Cr ed lp ra ne Ge y l og co gy On olo at on Ne Appropriateness of Vancomycin prescribing by surgical service 40% 35% 35% 30% 25% 25% 20% 15% Appropriate Inappropriate 10% 5% 0% gy lo ro U s tic as Pl ic ed op r la rth O cu as ov di ar C al er y en G er rg su ro eu N Inappropriate use according to type of Vancomycin therapy 80% 65% 70% 60% 50% 40% Inappropriate 30% 19% 20% 16% 10% 0% Specific therapy Empirical therapy Surgical prophylaxis Compare with Hong Kong study 76% 80% 65% 70% 60% 50% 40% 30% KKUH PWH 19% 16% 20% 10% 19% 5% 0% Specific therapy Empirical therapy Surgical prophylaxis Inappropriate use of Vancomycin according to the type of therapy Inappropriate use of Vancomycin classified by microorganism 60% 50% 50% 40% 30% 19% 23% 20% 10% Inappropriate 4% 4% 0% O o er th us s m is an rg cc co ro te En us re SA R au M us cc is id co m lo er hy id ap ep St us cc co lo hy ap St Order for Vancomycin serum level Not applicable 22% Yes 69% No 9% Trough 81% Peak&Trough 19% Vancomycin serum level outcome Subtherapeutic level 57% Toxic level 14% Therapeutic level 29% Vancomycin protocol Vancomycin serum level outcome 18% 16% Neonatology Oncology General pediatrics Critical care medicine Surgery Infectious disease Nephrology Cardiology 14% 12% 10% 8% 6% 4% 2% 0% Therapeutic SubToxic Level Level therapeutic Level Response to non-therapeutic level No response 48% No appropriate response Appropriate response 32% 20% Failure 75% Success 25% Achievement of therapeutic level after response Nephrotoxicity A decrease of >50 ml/min in creatinine clearance due to Vancomycin use is considered nephrotoxic. Estimated creatinine clearance (before and after receiving Vancomycin) t-test: Difference is not significant (P>0.05). Nephrotoxicity 1 10 Srcr before mg/dl 0.4 2 12 * 0.4 0.5 218 143.8 3 20 0.5 1.6 209.7 63.9 4 23 * 0.6 3.4 169 28.9 5 33 * 1.3 5.1 83.7 21 6 33 * 1.2 5.6 86.8 19 7 56 * 1 3.3 75.1 21.9 8 61 * 0.6 1.1 122.5 69.8 9 64 * 0.7 1.5 105 49 10 70 * 0.6 2.5 97.4 24 Mean ± S.D - 0.73 ± 0.32 2.51 ± 1.8 137 ± 57 57.9 ± 47.4 Number of patients Age (year) Srcr after mg/dl 0.5 Clcr befor ml/min 203 Clcr after ml/min 138 * Received Vancomycin with other nephrotoxic drugs. P < 0.05 Compare with Hong Kong study 25 21 20 17 Number of patients 15 10 10 Nephrotoxicity 8 5 0 KKUH PWH Cost The cost of Vancomycin during the study period 47,901.44 SR. The cost / therapeutic course = 187.85 SR. The cost of Vancomycin in which inappropriately used = 10,115 SR or (21%). = Conclusion 1) Vancomycin was inappropriately used in 125 patients (49%). 2) The most common situation in which the use of Vancomycin was inappropriate is continuing empirical use for presumed infections in patients whose cultures were negative for β-lactam resistant gram-positive organisms. Conclusion 3) ICU was the department with the highest inappropriate use of Vancomycin (55%). 4) Vancomycin serum level was sub-theraputic in 101 patients (57%). 5) Nephrotoxicity was found only in 10 patients (4.5%). Limitations • • • Retrospective Poor documentation Covered short period Recommendations 1) Development & distribution of practical guidelines for Vancomycin use. 2) Adhere to Vancomycin guidelines. 3) Implementation of programs: • Formal reassessment of therapy after 2-4 days. • Educational interventions & programs for physicians. • Computer-assisted prescribing of Vancomycin. • Clinical pharmacist in Infectious disease team. Recommendations 4) Continued evaluation of Vancomycin usage to ensure that interventions for improving its use are effective. Even 50 years after Vancomycin’s discovery, it remains an interesting and somewhat controversial agent Acknowledgements