* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Congenital_Heart_Dz
History of invasive and interventional cardiology wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Heart failure wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Artificial heart valve wikipedia , lookup
Myocardial infarction wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Aortic stenosis wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
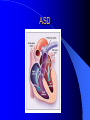
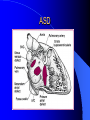
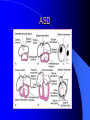
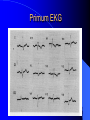
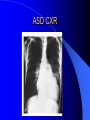
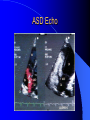
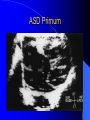
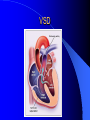
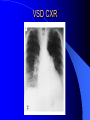
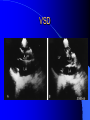
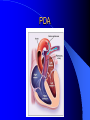
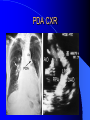
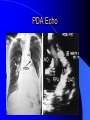
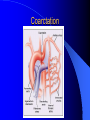
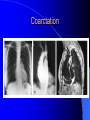
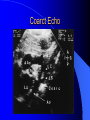
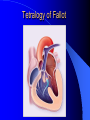
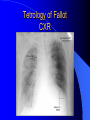
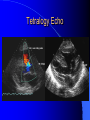
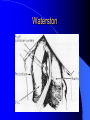
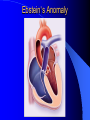
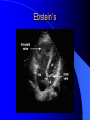
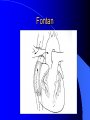
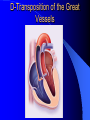
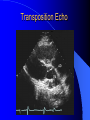
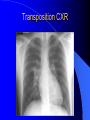
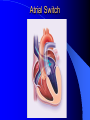
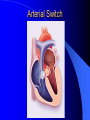
Congenital Heart Disease in Adults Background 8/1000 Live born births 32,000 cases/yr Liveborn prevalence lower than fetal prevalence – Fetal echo 20% die within first year – 80% of first year survivors reach adulthood – Prevalence 800,000 adults in U.S. Focus on Adult congenital heart disease Atrial Septal Defect One third of adult patients with CHD F:M=2:1 Secundum (75%) Primum 15% Sinus Venosus 10% ASD ASD ASD ASD Associated Abnormalities MVP Cleft Mitral Leaflet MR (Primum) Anomalous Pulmonary Venous Return – Sinus Venosus ASD Physiology Increased flow L-R – High-low pressure Increased Right sided blood flow Dilation of RA, RV and PA ASD Clinical Presentation No symptoms until third or fourth decades of life despite pulmonary to systemic flow (Qp:Qs) of 1.5 or more Over the years, the increased volume of blood usually causes right ventricular dilatation and failure Fatigue or dyspnea on exertion Supraventricular arrhythmias Paradoxical embolism, or recurrent pulmonary infections Death from RV failure or Arrhythmias in 40-50’s if uncorrected Physical Exam Right ventricular or pulmonary arterial impulse may be palpable. Wide and fixed splitting of the second heart sound – Increased blood flow in PA A systolic ejection murmur second left intercostal space (pulmonic) – usually so soft that it is mistaken for an “innocent”flow murmur. Flow across the atrial septal defect itself does not produce a murmur. ASD EKG Right-axis deviation Incomplete right bundle-branch block – R’ > R in V1 Left-axis deviation occurs with ostium primum defects – 1-deg AVB, “notched s wave II” A junctional or low atrial rhythm (inverted P waves in the inferior leads) occurs with sinus venosus defects. Normal sinus rhythm for the first three decades of life, after which atrial arrhythmias may appear. Secundum EKG Primum EKG ASD CXR Prominent pulmonary arteries Peripheral pulmonary vascular pattern – Small pulmonary arteries well visualized in periphery RAE/RVE when advanced ASD CXR ASD Echo RAE/RVE Direct visualization of Primum and Secundum defects Sinus venosus defects require TEE Microbubbles to assist with diagnosis ASD Echo ASD Primum ASD Treatment Qp:Qs 1.5 or more should be closed to prevent right ventricular dysfunction Not recommended if irreversible pulmonary hypertension Prophylaxis against infective endocarditis not recommended repaired or unrepaired – Except for first 6 months after closure Percutaneous Closure VSD VSD Most common congenital cardiac abnormality in infants and children M:F=1:1 25-40 percent close spontaneously by 2 y.o. 90 percent of those that eventually close do so by age10 70% are membranous, 20% muscular 5% just below the aortic valve (undermining the valve annulus and causing regurgitation), VSD Physiology Initially left-to-right shunting predominates Over time pulmonary vascular resistance increases and left-to-right shunting declines Eventually the pulmonary vascular resistance exceeds the systemic resistance and right to left shunting begins VSD Exam With left-to-right shunting and no pulmonary hypertension – left ventricular impulse is dynamic and laterally displaced – murmur is holosystolic, loudest at the lower left sternal border usually accompanied by a palpable thrill – A short mid-diastolic apical rumble (caused by increased flow through the mitral valve) may be heard VSD Exam Small, muscular VSD may produce high frequency systolic ejection murmurs that terminate before the end of systole – High pressure, small defect – defect is occluded by contracting heart muscle. If pulmonary hypertension develops, RV heave and a pulsation over the pulmonary trunk may be palpated – Murmur and thrill eventually disappear as flow through the defect decreases Cyanosis and clubbing are late findings VSD EKG Small defect-normal Large defect- left atrial and ventricular enlargement If pulmonary hypertension occurs – QRS axis shifts to the right, – right atrial and ventricular enlargement VSD CXR Small defect- normal Large defect LAE, LVE, “Shunt Vascularity” Pulmonary hypertension: – proximal pulmonary arteries enlarged – rapid taperingof the peripheral pulmonary arteries, and oligemic lung fields “Pruning” VSD CXR VSD Echo Two-dimensional echocardiography Confirm the presence and location Color-flow mapping provides information about the magnitude and direction of shunting Qp:Qs VSD VSD Management Small defects (Qp:Qs < 1.5) – No need for surgery – High Risk SBE, Prophylaxis provided Large defects who survive to adulthood usually have left ventricular failure or pulmonary hypertension/ right ventricular failure – Surgical closure recommended Once the ratio of pulmonary to systemic vascular resistance > 0.7 risk of surgery is prohibitive PDA PDA Connects descending aorta (just distal to the left subclavian artery) to the left pulmonary artery In the fetus, it permits pulmonary arterial blood to bypass lungs and enter the descending aorta for oxygenation in the placenta 10 percent of cases of congenital heart disease. – Perinatal hypoxemia – Maternal rubella – Infants born at high altitude or prematurely PDA Exam Bounding arterial pulses with widened pulse pressure Hyperdynamic left ventricular impulse A continuous “machinery” murmur – Second left anterior intercostal space – Peaks immediately after the second heart sound (thereby obscuring it) – declines in intensity during diastole. If pulmonary hypertension develops continuous murmur decreases in duration eventually disappears PDA CXR Left atrial and ventricular hypertrophy Pulmonary plethora, proximal pulmonary arterial dilatation, RVH Prominent ascending aorta May be visualized as an opacity at the confluence of the descending aorta and the aortic knob PDA CXR PDA Imaging With two-dimensional echocardiography the ductus arteriosus can usually be visualized Doppler studies demonstrate continuous flow in the pulmonary trunk Quantify the magnitude of shunting PDA Echo PDA Management Small defects – No need for surgery – High Risk SBE (0.45 % annually after age 20) Prophylaxis provided – Some recommend closure to prevent SBE Large defects – Sx during childhood or adulthood: fatigue, dyspnea, or palpitations – The ductus arteriosus may become aneurysmal and calcified, which may lead to its rupture – Left ventricular failure from Vol overload – When pulmonary vascular resistance exceeds systemic vascular resistance, the direction of shunting reverses (Cyanosis) PDA Surgery 1/3 of patients not surgically repaired die of heart failure, pulmonary hypertension, or endarteritis by age 40 2/3 die by age 60 Surgical ligation or percutaneous closure accomplished without cardiopulmonary bypass Mortality of less than 0.5 percent Once severe pulmonary vascular obstructive disease develops closure is contraindicated. Coarctation Coarctation Physiology A diaphragm-like ridge extending into aorta just distal to the left subclavian artery at the ligamentum arteriosum Less commonly immediately proximal to the left subclavian artery – difference in arterial pressure is noted between the arms Collateral circulation through the internal thoracic, intercostal, subclavian, and scapular arteries develops Coarctation M:F = 4-5:1 Associated abnormalities Gonadal dysgenesis (e.g.,Turner’s syndrome) Bicuspid aortic valve (30%) Ventricular septal defect Patent ductus arteriosus Mitral stenosis or regurgitation Aneurysms of the circle of Willis Coarctation Presentation Most adults are asymptomatic Diagnosis is made during physical exam – Systemic arterial hypertension observed in the arms, with diminished or absent femoral pulses If symptoms are present, they are usually those of hypertension: headache, epistaxis, dizziness, and palpitations. Occasionally, diminished blood flow to the legs causes claudication May present with heart failure or aortic dissection Women with coarctation are at high risk for aortic dissection during pregnancy Coarctation Physical Exam Systolic arterial pressure higher in the arms than in the legs The femoral arterial pulses are weak and delayed A systolic thrill in the suprasternal notch A systolic ejection click (due to a bicuspid aortic valve) A harsh systolic ejection murmur along the left sternal border and in the back, particularly over the coarctation A systolic murmur, caused by flow through collateral vessels, may be heard in the back Coarctation CXR Increased collateral flow through the intercostal arteries causes notching of the posterior third of the third through eighth ribs – Usually symmetric. Notching is not seen in the anterior ribs – Anterior intercostal arteries are not located in costal grooves The coarctation may be visible as an indentation of the aorta with prestenotic and poststenotic dilatation of the aorta, producing the “reversed E” or “3” sign Coarctation Coarctation Imaging The coarctation may be visualized echocardiographically Doppler examination can estimate transcoarctation pressure gradient. Computed tomography, magnetic resonance imaging, and contrast aortography – Location and length of the coarctation – Visualization of the collateral circulation – Measurement of Gradient on Cath Coarct Echo Coarctation Complications Hypertension Left ventricular failure (2/3 of pts > 40 yo) Aortic dissection Premature coronary artery disease Infective endocarditis Cerebrovascular accidents (due to the rupture of an intracerebral aneurysm) If uncorrected 3/4 die by the age of 50, and 90% by the age of 60 Coarctation Repair Repair considered for transcoarctation pressure gradient of more than 30 mm Hg Balloon dilatation is a therapeutic alternative – Higher incidence of subsequent aortic aneurysm and recurrent coarctation than surgical repair Postoperative complications include residual or recurrent hypertension, recurrent coarctation, and the possible sequelae of a bicuspid aortic valve Age at Time of Repair Surgery during childhood: – 90 percent are normotensive 5 years later, 50 percent are normotensive 20 years later – 89 percent of patients are alive 15 years later and 83 percent are alive 25 years later Surgery after age 40: – Half have persistent hypertension – 15-year survival is only 50 percent Bicuspid AoV Aortic Stenosis Supravalvular and Infravalvular Stenoses typically present in childhood Bicuspid aortic valve 2 to 3 percent adult population. M:F=4:1 20% have associated cardiovascular abnormality such as patent ductus arteriosus or aortic coarctation. Not stenotic at birth, subject to abnormal hemodynamic stress, leads to thickening and calcification of the leaflets Abnormality of the medial layer of the aorta above the Valve predisposes to dilatation of the aortic root Aortic Stenosis Presentation The classic symptoms are angina pectoris, syncope and heart failure Adults with aortic stenosis who are asymptomatic have a normal life expectancy; they should receive antibiotic prophylaxis Once symptoms appear, survival is limited: the median survival – five years after angina develops – three years after syncope occurs – two years after heart failure appears Aortic Stenosis Physical Exam Carotid upstroke delayed and diminished (parvus et tardus) The aortic component of S2 diminished or inaudible Fourth heart sound is present A harsh systolic crescendo–decrescendo murmur is audible over the aortic area and often radiates to the neck As the aortic stenosis worsens, the murmur peaks progressively later in systole Aortic Stenosis Work Up Left ventricular hypertrophy is usually evident on EKG Unless the left ventricle dilates, CXR demonstrates a normal cardiac silhouette TTE with Doppler permits assessment of the severity of the stenosis and of left ventricular systolic function. Cardiac catheterization is performed to determine the severity of aortic stenosis and to determine concomitant coronary artery disease. Aortic Stenosis Treatment If mild, only SBE prophylaxis If symptomatic, valve replacement necessary Valve replacement prior to development of LV dysfxn – Nl LV fxn – LVH will regress Pulmonic Stenosis 10 to 12 percent of congenital heart disease in adults. Valvular in 90 percent of patients, remainder supravalvular or subvalvular Supravalvular pulmonary stenosis in pulmonary trunk or branches – Often coexists with other congenital cardiac abnormalities (valvular pulmonary stenosis, ASD, VSD, PDA, tetralogy of Fallot or Williams syndrome) Subvalvular pulmonary stenosis caused by narrowing of the right ventricular infundibulum usually occurs in ventricular septal defect. Pulmonary Stenosis Physiology Typically is an isolated abnormality, may occur with VSD Valve leaflets usually are thin and pliant; all three valve cusps are present Commissures are fused – Valve is dome-shaped with a small central orifice – 10-15 percent have dysplastic thickened leaflets 2/3 of patients with Noonan’s syndrome have pulmonary stenosis due to valve dysplasia. Pulmonic Stenosis Definition Mild if the valve area >1.0 cm, transvalvular gradient < 50 mm Hg, or peak right ventricular systolic pressure is <75 mm Hg Moderate if the valve area is 0.5 to 1.0 cm, the transvalvular gradient is 50 to 80 mm Hg, or the right ventricular systolic pressure is 75 to 100 mm Hg. Severe pulmonary stenosis is characterized by a valve area of less than 0.5 cm, a transvalvular gradient of > 80 mm Hg, or a right ventricular systolic pressure of more than 100 mm Hg Pulmonic Stenosis Presentation If mild, usually Asx When the stenosis is severe, dyspnea on exertion or fatigability may occur Less often may have chest pain or syncope with exertion Eventually, right ventricular failure may develop, with peripheral edema and abdominal swelling If the foramen ovale patent, shunting of blood from the right to the left causing cyanosis and clubbing Pulmonic Stenosis Physical Exam With moderate or severe pulmonary stenosis: A right ventricular impulse at the left sternal border Thrill at the second left intercostal space Harsh crescendo–decrescendo systolic murmur increases with inspiration at left sternal border If the valve is pliable, an ejection click often precedes the murmur As the stenosis becomes more severe, the systolic murmur peaks later in systole Pumonic Stenosis CXR Post-stenotic dilatation of the main pulmonary artery Diminished pulmonary vascular markings The cardiac silhouette is usually normal – An enlarged cardiac silhouette may be seen if the patient has right ventricular failure or tricuspid regurgitation. Pulmonic Stenosis Echo Right ventricular hypertrophy and paradoxical septal motion during Site of obstruction can be visualized in most patients. With the use of Doppler flow studies, the severity of stenosis can usually be assessed Pulmonic Stenosis Echo Pulmonic Stenosis, Treatment If mild only SBE Prophylaxis Survival 94 percent 20 years after diagnosis Severe stenosis should be relieved Moderate pulmonary stenosis have an excellent prognosis with either medical or interventional therapy – Interventional therapy is usually recommended, since most patients with moderate pulmonary stenosis eventually progress Balloon Valvuloplasty The procedure of choice High success rate provided the valve is mobile and pliant Long-term results are excellent Secondary hypertrophic subpulmonary stenosis regresses after successful intervention Valve replacement is required if the leaflets are dysplastic or calcified or if marked regurgitation is present Tetrology of Fallot Most common cyanotic heart defect after infancy Overiding aorta Obstruction of RVOT RVH VSD Associated with L-PA stenosis (40%), R sided aortic Arch (25%), ASD (10%), Coronary Anomalies (10%) Tetralogy of Fallot Tetralogy of Fallot Equal pressure in R and L ventricles R-L shunting due to elevated RV pressures from RVOT obstruction Changes in SV resistance affect shunting – Increased SVR decreases R-L shunting Tetralogy of Fallot Presentation Cyanotic spells beginning in first year of life – Tachypnea, cyanosis – Can progress to LOC, Seizures, CVA, Death Adults – Dyspnea and limited exercise tolerance – Complications of chronic cyanosis- erythrocytosis, hyperviscosity, abnormalities of hemostasis, cerebral abscesses or stroke, and endocarditis. Tetralogy of Fallot Physical Exam Cyanosis and digital clubbing – Severity determined by the degree of RVOT obstruction RV lift is palpable A Systolic ejection murmur caused by turbulent flow across the RVOT (thrill may be may be palpable) – Intensity and duration inversely proportional to severity of obstruction- flow shunted across VSD – a soft, short murmur suggests severe obstruction Second heart sound is single, since its pulmonary component is inaudible An aortic ejection click (due to a dilated, overriding aorta) may be heard Tetralogy of Fallot EKG- right-axis deviation and right ventricular hypertrophy. CXR- heart size is normal or small – lung markings are diminished. – “bootshaped,” heart – upturned right ventricular apex and concave main pulmonary arterial segment. – A right sided aortic arch may be present. Tetrology of Fallot CXR Tetralogy EKG Tetralogy of Fallot Echo Establishes diagnosis Determines severity of RVOT obstruction Flow across VSD Cardiac Cath – Pressures, gradients, shunting, O2 sat, VSD – Origins of coronary arteries Also seen by MRI or CTA Tetralogy Echo Tetralogy of Fallot Without surgical intervention, most patients die in childhood Survival rate- 66 percent at 1 year of age, 40 percent at 3 years, 11 percent at 20 years, 6 percent at 30 years, and 3 percent at 40 years Tetralogy of Fallot Surgical correction Relieves sx and improves survival Waterston: a side-to-side anastomosis of the ascending aorta and the right pulmonary artery Potts: side-to-side anastomosis of the descending aorta to the left pulmonary artery Blalock–Taussig: end-to-side anastomosis of the subclavian artery to the pulmonary artery. – Long-term complications- pulmonary hypertension, left ventricular volume overload, and distortionof the pulmonary arterial branches. Blalock-Tausig Waterston Tetralogy of Fallot Surgical correction Complete surgical correction – Closure of VSD – Relief of RVOT obstruction Mortality 3% in children, 2.5-8% in Adults Rate of survival 32 years after surgery 86% with repair vs. 96% in agematched controls Tetralogy of Fallot Post Surgical Complications Ventricular arrhythmias detected with Holter monitoring in 40 to 50 percent Moderate or severe pulmonary regurgitation Systolic and diastolic ventricular dysfunction Atrial fibrillation or flutter are common Tetralogy of Fallot Post Surgical Complications Pulmonary regurgitation may develop as a consequence of surgical repair of the RVOT – Can result in RVE and RV dysfunction – May require repair or replacement of the pulmonary valve RVOT aneurysm may occur at site of repair – Rupture has been reported Recurrent obstruction of RVOT may occur 10-20% have residual VSD CHB may occur AI is common but usually mild Ebstein’s Anomaly Downward displacement of septal leaflet of Tricuspid valve – Sometime posterior leaflet as well “Atrialized Ventricle” Tricuspid regurg common 80% have ASD or PFO – Can result in R-L shunting Ebstein’s Anomaly Ebstein’s Ebstein’s Anomaly Severity of defect depends upon degree of valvular displacement Presentation ranges from severe HF in neonate to incidental discovery in adults Neonates with severe disease have cyanosis, heart failure, murmur noted in the first days of life – Worsens after the ductus arteriosus closes Older children with Ebstein’s anomaly often come to medical attention because of an incidental murmur Adolescents and adults present with a supraventricular arrhythmia. Ebstein’s Anomaly Physical Exam Severity of cyanosis depends on the magnitude of right-to-left shunting Tricuspid regurgitation is usually present at the left lower sternal border. Hepatomegaly from passive hepatic congestion due to elevated right atrial pressure may be present. Ebstein’s Anomaly EKG Tall and Broad p-waves RBBB 1st degree AVB 20% have ventricular pre-excitation Ebstein’s Anomaly EKG Ebstein’s Anomaly CXR Normal in mild cases Cardiomegally from RAE Pulmonary markings decreased in severe cases – Marked R-L shunting across ASD Ebstein’s Anomaly Treatment Focuses on preventing and treating complications SBE prophylaxis CHF Rx of SVT – RFA for accessory pathway Fontan procedure in severe cases Fontan Ebstein’s Anomaly Tricuspid Surgery Repair or replacement Closure of ASD/PFO Patient with severe sx despite medical Rx Cardiac enlargement Transposition of the Great Vessels Aorta from RV, PA from LV Complete separation of pulmonic and arterial saturation Requires communication between the circuits for survivial – PDA, VSD, ASD or PFO D-Transposition of the Great Vessels Transposition Echo Transposition of the Great Vessels Physical Exam Findings are nonspecific. Infants have cyanosis and tachypnea. The second heart sound is single and loud (due to the anterior position of the aorta). In patients with mild cyanosis, a holosystolic murmur caused by a ventricular septal defect may be heard. A soft systolic ejection murmur (due to pulmonary stenosis, ejection into the anteriorly located aorta, or both) may be audible. Transposition of the Great Vessels EKG RAD RVH- RV is systemic ventricle LVH- if VSD, PDA, Pulmonic Stenosis present Transposition of the Great Vessels CXR Increased pulmonary vascularity Egg Shaped with a narrow stalk Transposition CXR Transposition of the Great Vessels Mortality 90% by 6 months if uncorrected Infusion of prostaglandin E (to maintain or restore patency of the ductus arteriosus), Creation of an atrial septal defect by means of balloon atrial septostomy (the Rashkind procedure). Oxygen- to decrease PVR, increase pulmonary blood flow Transposition of the Great Vessels-Surgery Atrial Switch- (Mustard) – Atrial septum excised and baffle created – Shunts blood to LV RV continues to function as systemic ventricle – RV failure, SCD Leakage of the atrial baffle (often clinically inconsequential) Obstruction of the baffle (often insidious and frequently asymptomatic) Sinus-node dysfunction Atrial arrhythmias, particularly atrial flutter Atrial Switch Arterial Switch Transposition of the Great Vessels-Surgery The atrial-switch operation has been replaced by the arterial-switch operation Pulmonary artery and ascending aorta are transected above the semilunar valves Coronary arteries switched, so that the aorta is connected to the neoaortic valve (formerly the pulmonary valve) arising from the left ventricle, and the pulmonary artery is connected. This operation can be performed in neonates and is associated with a low operative mortality and an excellent long-term outcome. Physiologic Repair Tetralogy of Fallot (TOF) Senning's or Mustard's operation for transposition of the great arteries Fontan operation for the single ventricle. Approach to Management Timetable of Congential Heart Surgery Congenital Heart Disease in Adults Part II Cyanotic Heart Disease M.Ferguson CAPT, USN NNMC Palliative interventions increase or decrease pulmonary blood flow while allowing a mixed circulation and cyanosis to persist Physiologic repair total or near total anatomic, physiologic, or both anatomic and physiologic separation of the pulmonary and systemic circulations. Palliative Operations Palliative Operations Systemic arterial-to-pulmonary artery shunts – improvement in saturation levels – high levels of pulmonary blood flow – direct exposure of the pulmonary vascular bed to the high pressures of the systemic circulation – long-term complications include pulmonary hypertension, pulmonary artery stenosis, and volume overload of the ventricle receiving pulmonary venous return. Cyanotic Conditions Arterial O2 desaturation due to shutning of venous blood into arterial circulation (R-L) Magnitude of shunting determines severity of desaturation