* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Orbits and Orbitals
Quantum electrodynamics wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Quantum machine learning wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Quantum key distribution wikipedia , lookup
Franck–Condon principle wikipedia , lookup
History of quantum field theory wikipedia , lookup
Bell's theorem wikipedia , lookup
Quantum group wikipedia , lookup
Canonical quantization wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Wave–particle duality wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Spin (physics) wikipedia , lookup
Coupled cluster wikipedia , lookup
Particle in a box wikipedia , lookup
Quantum state wikipedia , lookup
Hidden variable theory wikipedia , lookup
EPR paradox wikipedia , lookup
Chemical bond wikipedia , lookup
Atomic theory wikipedia , lookup
Hartree–Fock method wikipedia , lookup
Ferromagnetism wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Tight binding wikipedia , lookup
Hydrogen atom wikipedia , lookup
Molecular orbital wikipedia , lookup
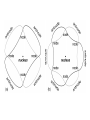
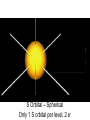
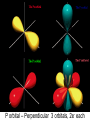
Orbits and Orbitals Basic Bohr to Quantum Orbitals KLM to spdf KLM and spdf??? • KLM – Bohr style / old style lettering for the “shells” or “orbits”. K is closest to the nucleus, L is next, M (N,O,P etc) • Now we know that KLM is “really” the “Principle Quantum Number” 1,2,3 for the energy levels • spdf – refers to the shape of the “orbital” – A short deBroglie diversion More rules • No orbital can have more than 2 e- in it. (one spin up, one spin down) • Orbitals are half filled (with spins in the same direction) before they are doubly filled. • Orbitals are filled from lowest energy to highest energy. Confusing? • OK • “Quantum Numbers” are the way we can describe locations where electrons can be found. • The “Principle Quantum Number” – n – is the same as the old shells, and is the same as the number of the Period on the table. n, l, m, s • n = energy level (old shell / Bohr model) • l = angular momentum => shape – – s = 0 , p = 1, d = 2, f = 3 l≤n-1 • m = magnetic quantum number => orientation – -l ≤ m ≤ +l • s = spin ( +½, -½ or: up, down) That didn’t Help!!! • In the 1st level – Only an s orbital with 2electrons • In the 2nd level, an s orbital with 2 electrons and a p orbital with 6 electrons • OK, pictures are easier S Orbital – Spherical Only 1 S orbital per level, 2 e- P orbital – Perpendicular 3 orbitals, 2e- each D Orbitals – “double dumbbells” 5 orbitals, 2e- each, 10e- total F orbital – “dead spiders” 7 orbitals – 2e- in each – 14 total *late breaking info – the above is correct, however since I went to school, a new shape – the one in the middle, has been added Now, How do you Fill Them • You fill the orbitals from the lowest energy on up – Half filling them first, (with spins the same) then doubly filling them Aufbau principle 1s *we haven’t needed these yet 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 5g* 6s 6p 6d 6f*6g*6h* 7s 7p Some practice – Some Questions • Look up the Atomic number, and then determine (not look up) the electron orbital configuration. • H He • O K • Ca Cr • U Ag • Au Pt Compare your configuration for Cr, Ag, Au and Pt with the “official” one. Are they the same? If not, why are they different? Noble Gas Configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6 He Ne Ar Kr Xe Rn Uuo Olympicene Pentacene 5 linked carbon rings with hydrogens Fullerenes Magic Numbers 2 – 8 – 8 – 18 -18 2 – 6 – 10 – 14 (1,3,5,7) electronegativity Disclaimer Aloha I put together these power points for use in my science classes. You may use them in your classes. Some images are public domain, some are used under the fair-use provisions of the copyright law, some are mine. Copyright is retained by the owners! Ted Brattstrom