* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PowerPoint - Balancing Equations
Inorganic chemistry wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Safety data sheet wikipedia , lookup
Process chemistry wikipedia , lookup
Chemical element wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemical plant wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical potential wikipedia , lookup
Biochemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Chemical industry wikipedia , lookup
Rate equation wikipedia , lookup
Computational chemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Drug discovery wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical bond wikipedia , lookup
Transition state theory wikipedia , lookup
History of chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
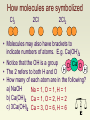
Chemical Equations Their Job: Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O2 (g) 2 Al2O3 (s) The numbers in the front are called Stoichiometric Coefficients_ The letters (s), (g), (l) and (aq) are the physical states of compounds. Introduction – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe a chemical reaction Parts of a Reaction Equation – Chemical equations show the conversion of reactants (the molecules shown on the left of the arrow) into products (the molecules shown on the right of the arrow). • A “+” sign separates molecules on the same side • The arrow () is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen react to yield carbon dioxide” Chemical Equations Because of the principle of the conservation of matter, an equation must be balanced. It must have the same number of atoms of the same kind on both sides. Lavoisier, 1788 Symbols Used in Equations • Solid __(s)_ • Liquid (l) • Gas _(g)__ • Aqueous solution (aq) How molecules are symbolized Cl2 2Cl 2Cl2 • Molecules may also have brackets to indicate numbers of atoms. E.g. Ca(OH)2 • Notice that the OH is a group O Ca O H • The 2 refers to both H and O H • How many of each atom are in the following? a) NaOH Na = 1, O = 1, H = 1 b) Ca(OH)2 Ca = 1, O = 2, H = 2 c) 3Ca(OH)2 Ca = 3, O = 6, H = 6 Balancing Equations – When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. • Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent) Subscripts vs. Coefficients • The subscripts tell you how many atoms of a particular element are in a compound. The coefficient tells you about the quantity, or number, of molecules of the compound. Balancing equations: MgO • The law of conservation of mass states that matter can neither be created or destroyed • Thus, atoms are neither created or destroyed, only rearranged in a chemical reaction • Thus, the number of a particular atom is the same on both sides of a chemical equation • Example: Magnesium + Oxygen (from lab) • Mg + O2 MgO Mg + O O Mg O • However, this is not balanced • Left: Mg = 1, O = 2 • Right: Mg = 1, O = 1 Balance equations by “inspection” From Mg + O2 MgO 2Mg + O2 2MgO Mg + ½O2 MgO Mg2 + O2 2MgO 4Mg + 2 O2 4MgO is correct is incorrect is incorrect is incorrect Hints: start with elements that occur in one compound on each side. Treat polyatomic ions that repeat as if they were a single entity. a) P4 + 5O2 P4O10 b) 2Li + 2H2O H2 + 2LiOH c) 2Bi(NO3)3 + 3K2S Bi2S3 + 6KNO3 d) C2H6 + 3.5O2 2CO2 + 3H2O 2 C2H6 + 7 O2 4 CO2 + 6 H2O K + H2O H2 + KOH • Reactants • Products •K •K •H •H •O •O Steps to Balancing Equations There are four basic steps to balancing a chemical equation. Step 1: 1. Write the correct formula for the reactants and the products. DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. And most importantly, once you write them correctly DO NOT CHANGE THE FORMULAS! Step 2: 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. Step 3: 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. Step 4: 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. (reduced) Some Suggestions to Help You Some Helpful Hints for balancing equations: • Take one element at a time, working left to right except for H and O. Save H for next to last, and O until last. • IF everything balances except for O, and there is no way to balance O with a whole number, double all the coefficients and try again. (Because O is diatomic as an element) Balance these skeleton equations: a) b) c) d) e) f) g) h) i) Mg + HCl MgCl2 + H2 Ca + N2 Ca3N2 NH4NO3 N2O + H2O BiCl3 + H2S Bi2S3 + HCl C4H10 + O2 CO2 + H2O O2 + C6H12O6 CO2 + H2O NO2 + H2O HNO3 + NO Cr2(SO4)3+ NaOH Cr(OH)3+ Na2SO4 Al4C3 + H2O CH4 + Al(OH)3 Balance these skeleton equations: a) b) c) d) e) f) g) h) i) Mg + 2HCl MgCl2 + H2 3Ca + N2 Ca3N2 NH4NO3 N2O + 2H2O 2BiCl3 + 3H2S Bi2S3 + 6HCl 2C4H10 + 13O2 8CO2 + 10H2O 6O2 + C6H12O6 6CO2 + 6H2O 3NO2 + H2O 2HNO3 + NO Cr2(SO4)3+ 6NaOH 2Cr(OH)3+ 3Na2SO4 Al4C3 + 12H2O 3CH4 + 4Al(OH)3 a) b) c) d) e) f) g) h) Li + H2O H2 + LiOH P4 + O2 P4O10 C2H6 + O2 CO2 + H2O CS2 + O2 CO2 + SO2 AsCl3 + H2S As2S3 + HCl AgNO3 + FeCl3 AgCl + Fe(NO3)3 KClO3 KCl + O2 SO2 + O2 SO3 a) b) c) d) e) f) g) h) 2Li + 2H2O H2 + 2LiOH P4 + 5O2 P4O10 2C2H6 + 7O2 4CO2 + 6H2O CS2 + 3O2 CO2 + 2SO2 2AsCl3 + 3H2S As2S3 + 6HCl 3AgNO3 + FeCl3 3AgCl + Fe(NO3)3 2KClO3 2KCl + 3O2 2SO2 + O2 2SO3