* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Using Transgenic Technology to Characterize Regulatory Regions
Genetic engineering wikipedia , lookup
Gene nomenclature wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Minimal genome wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Genomic imprinting wikipedia , lookup
Gene desert wikipedia , lookup
Genome (book) wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Genome evolution wikipedia , lookup
Gene expression programming wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene expression profiling wikipedia , lookup
Microevolution wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
History of genetic engineering wikipedia , lookup
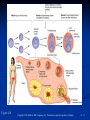
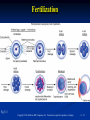
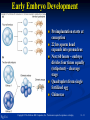
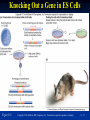
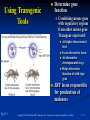
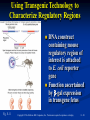
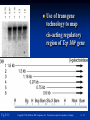
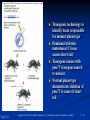
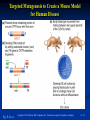
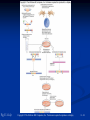
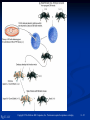
PowerPoint to accompany Genetics: From Genes to Genomes Fourth Edition Hartwell ● Hood ● Goldberg ● Reynolds ● Silver Reference E Prepared by Malcolm Schug University of North Carolina Greensboro Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-1 Mus musculus: Genetic Portrait of the House Mouse Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-2 Fig. E.1 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-3 Outline of Reference E Overview of Mus musculus in laboratory Mouse genome Mouse life cycle Transgenic protocols Uses of transgenic technology to determine Addition of genes by nuclear injection Removal of genes by targeted mutagenesis Function of gene products Characterizing regulatory regions Links between mutant phenotypes and transcription units Creating a mouse model for human disease The Hox genes: a comprehensive example Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-4 The Mouse Genome Similar to humans Contains about 3 billion nucleotides Homologues for every gene in humans Differences due to species-specific additions to gene families 19 autosomes and 2 sex chromosomes No evidence of banding similarities with humans in karyotypes Conserved synteny Genes closely linked in mouse are also closely linked in humans Average size of conserved syntenic regions: 17.6 Mb Since common ancestry, human and mouse genomes have broken apart and rearranged ~ 170 times May identify a gene in one species and locate it in other species Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-5 Table E.1 Comparison of Mice and Humans Trait Mice Humans Average weight 30g 77,000 g (170 lb) Average length 10 cm (without tail) 175 cm Genome size 3,000,000,000 bp 3,000,000,000 bp Haploid gene number 25,000 25,000 Number of chromosomes 19 autosomes + X and Y 22 autosomes + X and Y Gestation period 3 weeks 38 weeks Age at puberty 5-6 weeks 624-728 weeks Estrus cycle 4 days 28 days Life span 2 years 78 years Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-6 Conserved Synteny Fig. E.3 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-7 Life Cycle Fig. E.4 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-8 Germ Cell Development Males Sperm Fig. 4.21 At puberty, large number of haploid gametes produced for rest of life Spermatogenesis Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E-9 Germ Cell Development Female Born with all gametes they will ever have ~ 50,000 eggs, or oocytes Oogenesis begins in ovaries of fetus Oogonia enter meiosis but stop at diplotene in first prophase Estrus cycle begins at puberty 4 days in mice 8-10 primary oocytes complete first meiotic division Secondary oocyte stops at metaphase are released from ovary (ovulation) Oocyte passed into oviduct and is receptive to fertilization (estrus) Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 10 Figure 4.18 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 11 Fertilization Fig. E.5 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 12 Early Embryo Development Fig. E.6 Preimplantation starts at conception 22 hrs sperm head expands into pronucleus Next 60 hours – embryo divides four times equally (totipotent) – cleavage stage Quadruplets from single fertilized egg Chimeras Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 13 Events Restricting the Developmental Potency of Individual Cells Occur Near the End of the Preimplantation Stage 16-cell embryo First differentiation events occur Trophectoderm layer – cells on outside of embryo Inner cell mass – cells on inside of embryo Fetus is derived from ICM Blastocyte forms After implantation, placenta develops, embryo grows, and tissues and organs emerge Birth at 21 days after conception Suckling period lasts 18-25 days Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 14 Two Powerful Transgenic Techniques Addition of genes by nuclear injection Foreign DNA injected into pronucleus of fertilized egg Place injected one-cell embryo back into oviduct 25-50% of time DNA integrates at random into chromosome Targeted mutagenesis Embryonic stem lines divide without differentiating Cultures of thousands; some of which will be injected back into blastocoele Integrated into germ line Homologous recombination may occur after transfection Knockout constructs have nonfunctional gene that can be exchanged for mouse gene by homologous recombination Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 15 How Transgenic Mice are Created Fig. E.7 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 16 Knocking Out a Gene in ES Cells Figure E.8 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 17 Using Transgenic Tools Determine gene function Combining mouse gene with regulatory regions from other mouse gene Transgene expressed: Fig. E.9 At higher than normal level In an alternative tissue At alternative developmental stage Helps determine function of wild-type gene SRY locus responsible for production of maleness Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 18 Application of Transgenic Technology Fig. E.10 Transgenic expression of myc gene provides information on gene’s role in tumor formation (a) structure of gene (b) northern blot analysis Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 19 Using Transgenic Technology to Characterize Regulatory Regions Fig. E.11 DNA construct containing mouse regulatory region of interest is attached to E. coli reporter gene Function ascertained by b-gal expression in transgene fetus Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 20 Fig. E.12 Use of transgene technology to map cis-acting regulatory region of Tcp 10bt gene Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 21 Fig. E.13 Transgenic technology to identify locus responsible for mutant phenotype Dominant deletion mutation at T locus causes short tail Transgene mouse with pme75 transgene mated to mutant Normal phenotype demonstrates deletion of pme75 is cause of short tail Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 22 Targeted Mutagenesis to Create a Mouse Model for Human Disease Fig. E.14 a-c Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 23 Fig. E.14 d,e Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 24 Fig. E.14 f Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 25 Inheritance Pattern Fig. E.14g-h Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 26 The Hox Genes: A Comprehensive Example Homeotic selector genes (Hox) 4 gene clusters with 9-11 genes each Control development of body segments Used Hox genes of Drosophila as probes in cross-hybridization studies In flies, homeotic genes active in the discrete segments that define body plan, where they determine proper differentiation of tissues Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 27 Mouse Hox Gene Superfamily Contains Multiple Homologs of Each Member of the Drosophila Homeotic Selector Gene Family Fig. E.15 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 28 Analysis of Expression Patterns in Developing Embryos Provides Clue to Time and Location of Gene Action Spatial extent of expression in some Hox genes along developing spine Fig. E.16 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 29 Validating the Hypothesis that Expression of the 5 Gene in a Hox Cluster is Epistatic to Expression of the More 3 Genes Fig. E.17 Transgenic construct misexpresses HoxD4 in more anterior region where HoxA1 is normally located Skeleton of wild-type (left) and transgenic animal (right) Enlargement of cervical regions from skeletons shown below Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 30 Knockout Studies Confirmed Predictions of Aberrant Phenotypes 5 epistasis hypothesis leads to prediction that aberrant phenotypes will arise when different Hox genes are knocked out by homologous recombination Data using knockouts support this hypothesis e.g., knockout of HoxB4 produces a partial homeotic transformation of second cervical vertebra from axis to atlas Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 31 Fig. E.16 Copyright © The McGraw-Hill Companies, Inc. Permission required to reproduce or display E - 32