* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download IMMUNOLOGY ADVANCED
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
History of genetic engineering wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene expression profiling wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genetic engineering wikipedia , lookup
Population genetics wikipedia , lookup
Medical genetics wikipedia , lookup
Gene therapy wikipedia , lookup
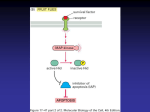
Gene therapy of the human retina wikipedia , lookup
Public health genomics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Designer baby wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Genome (book) wikipedia , lookup
Frameshift mutation wikipedia , lookup
Oncogenomics wikipedia , lookup
IMMUNOLOGY ADVANCED: THE GENETIC PATHOPHYSIOLOGY OF PRIMARY IMMUNODEFICIENCY DISORDERS (PIDs) Dr. Peter Vickers 23/05/2017 1 CONTENTS Introduction Genetic classification of PIDs Antibody deficiencies: 1. XLA Antibody deficiencies: 2.CVID Antibody deficiencies: 3. SIgA T-cell/combined deficiencies – ADA SCID Summary References & Further reading 23/05/2017 2 INTRODUCTION PADs can be classified as being caused by: 1. a single gene mutation (e.g. XLA) 2. a multi-genetic susceptibility (e.g. CVID) The 3 main modes of genetic inheritance are: Autosomal recessive Autosomal dominant X-linked recessive although there are other ways of obtaining genetic defects, e.g. spontaneous mutation . 23/05/2017 3 Within these 3 modes of inheritance, genetic problems can be further subdivided by mutations within genes responsible for certain tasks, for example: Some PIDs are caused by mutations within the genes that are necessary for the development of a functionally mature cell line – for example Btk which is necessary for the development of B-cell lymphocytes 23/05/2017 4 Other mutations are caused by mutations in genes that are responsible and necessary for the development and functioning of a single precursor A third grouping of genetic mutations occurs when a gene that is expressed in multiple types of tissues/cells becomes mutated. 23/05/2017 5 Goals of Common Disease Genetics Phenotype Genotype • Work out the distribution of diseases or traits within families and across populations. • Identify the genes that predispose to a disease or phenotype • Determine if the particular diseases or intermediate phenotypes have a genetic component. • Understand how those genes work towards predisposition (e.g. interaction). • Describe the pre-clinical natural history • Identify the interactions between the gene and the environment WHY? We can better understand the disease pathogenesis We can identify the high risk populations This allows us to: •Develop specific preventive and therapeutic measures •Apply individualized medicine 23/05/2017 Adapted from Rotter 2004 6 GENETIC CLASSIFICATION OF PIDs Things are complicated by the fact that a specific clinical phenotype may occur as a result of mutations within multiple genes, which may sometimes occur with different modes of inheritance. An example of this is severe combined immunodeficiency (SCID): ◦ X-linked SCID is caused by mutations of the common gamma chain (γ-chain) ◦ Recessive SCID is caused by mutations of the JAK3 gene. However, both types clinically present as T- B+ NK- SCID. 23/05/2017 7 Another consideration is that mutations of the same gene can cause strikingly different clinical phenotypes. This is dependent upon the type of mutation and its position within the gene. Example: RAG-1/RAG-2 mutations usually result in T- B- NK+ SCID, but a different type of mutation within the gene – for example a missense mutation, which still allows the expression of a mutated gene (as opposed to a nonsense mutation which stops the production and expression of a gene) may result in Omenn Syndrome (T+ B- NK+), with the added clinical symptoms of erythematous rash, raised serum IgE, and eosinophilia. 23/05/2017 8 The fact that there are such a large number of genes that, if mutated, are linked to PIDs just serve to demonstrate how complex the immune system is. For example, genes involved in the maturation of lymphocyte subsets, such as Btk, RAG-1 and RAG-2 are responsible for immune cytopaenias and resulting immune defects, whilst mutations of genes involved in CD40 cell signalling cause problems with the signals that are sent out by various cells - and so on. In terms of the most common PIDs and genetic mutations, a large proportion of them have an X-linked inheritance, many have an autosomal recessive mode of inheritance, whilst a few are inherited via the autosomal dominant route. 23/05/2017 9 So it can be seen that genetically, the immune system is very prone to many and varied mutated genes, just because so many genes are involved in our immune system, which can lead to the many and variable presentations of PIDs. This session will look at just 4 of the many different PIDs so far identified in order to show how a PID can develop. This is important because it is only by an understanding of the genetics of PIDs and, in particular, the actual mutations that occur within the genes responsible for the immune system, that we can truly be able to treat/cure /care for children and adults with these disorders. 23/05/2017 10 X-LINKED AGAMMAGLOBULINAEMI A (Bruton’s Disorder) B-CELL DEFICIENCY 23/05/2017 11 The gene responsible for XLA is called Bruton's tyrosine kinase (Btk) This is a member of a family called Tec kinases, and Btk is a protein tyrosine kinase This protein is expressed in both B cells and neutrophils, but in XLA only the B cells are defective It is also expressed in erythroid precursors, myeloid cells, mast cells, monocytes, megakaryocites and platelets – but it is NOT expressed in T-cells and NK-cells. 23/05/2017 12 It is required for both B-cell survival and activation. This gene provides instructions for making the BTK protein, which is important for the development of B cells and normal functioning of the immune system. Mutations in the gene for Btk prevent the B cells from maturing because Btk is a crucial signalling molecule which regulates the development of B cells into plasma cells Only cells in which the normal allele of Btk is active can develop into mature B cells Thus, those people with defective Btk have impaired B cell development and poor or absent antibody production 23/05/2017 13 Most mutations in the Btk gene prevent the production of any BTK protein. The absence of functional BTK protein blocks B cell development and leads to a lack of antibodies. Signalling through the pre-B-cell receptors and B-cell antigen receptors controls these developmental stages and transitions. Consequently, in Btk-deficient cells, these processes do not happen and so the affected cells fail to proliferate, and are destroyed through apoptosis (cell suicide). Thus, in XLA patients, mature B-cells generally make up only about 0.1% of circulating lymphocytes. Typically about 1/3 of X-linked PIDs are new, sporadic mutations 23/05/2017 14 Genetics of X-linked Agammaglobulinaemia There are more than 300 known mutations and most of them cause a truncation of the protein. These mutations are either nonsense mutations or frameshift mutations (caused by insertions, deletions or splice site defects) (Vihinen M 2003) 23/05/2017 15 Clinical Features & Pathophysiology: Immunoglobulin G (IgG) is actively transported across the placenta, so XLA patients will generally have normal levels of IgG at birth and few, if any symptoms. Soon, though, the maternal IgG becomes catabolised, leading to hypogammaglobulinaemia and an increased susceptibility to infections. Bruton’s disorder – boys with Bruton’s usually present with recurrent pyogenic infections (fevers) between the ages of 4 months and 2 years. 23/05/2017 16 Over 90% of these infections will be in the respiratory tract, with 20% of them affecting the gastro-intestinal tract, 13% the central nervous system, 5% the genito-urinary tract, 5% the bones, and 9% affecting the skin and other organs. A medical history will usually show that the boy has had mainly recurrent infections of the upper and lower respiratory tract, whilst other usual presenting sites may well include: 23/05/2017 17 Skin sepsis – boils, abscesses and cellulitis Urinary tract infections Episodes of diarrhoea and vomiting Arthritis Meningitis ◦ with the commonest infecting agents being pyogenic bacteria (e.g. streptococci, Haemophilus influenza and Streptococcus pneumonia, although they may also have presented with less common infectious organisms. 23/05/2017 18 However, because cell-mediated immunity (Tcells) is still present in these boys, then they tend not to have problems with viral or fungal infections – but be aware that continual bacterial infections may eventually leave the body open to secondary viral and/or fungal infections. Laboratory analysis will tend to demonstrate absent or very few circulating mature B-cells, with normal or increased T-cells. 23/05/2017 19 Thus, the diagnosis of an antibody deficiency (not just XLA) is made from: Medical history Signs & Symptoms Low serum levels of all immunoglobulin classes Absence of mature circulating B-cells Family history of affected male relatives ( particularly when diagnosing Bruton’s XLA) A warning: in very exceptional circumstances and very rarely, female siblings of affected male children could also be antibody deficient. 23/05/2017 20 COMMON VARIABLE IMMUNODEFICIENCY (CVID) “CVID is currently viewed as a heterogeneous group of disorders with an intrinsic B-cell defect or a B-cell dysfunction related to abnormal T-cell-B cell interaction. In the majority of cases, there is no family history of a related or similar defect. However, in 10% to 20% of families, another member may have selective IgA deficiency, IgG deficiency, or much more rarely, CVID” (Ochs et al. 2004 page 375) 23/05/2017 21 CVID is often a ‘catch-all’ category for a group of immune deficiency disorders presenting in either childhood or adulthood – but the majority of people with this condition are usually not diagnosed until they reach adult status. So, CVID is an immune deficiency disorder that can present at any age, and is characterised by: ◦ Recurrent bacterial infections ◦ Hypogammaglobulinaemia ◦ Impaired antibody responses (even though B-cells are usually present) ◦ Normal/near normal T-cell immunity. 23/05/2017 22 Because the genetic elements of this disease are unpredictable and the fact that there is so much variability in age of onset, as well as the clinical and laboratory anomalies, it is believed that CVID is not caused by a single genetic defect. To date, no precise molecular defect has been identified for most cases of CVID (Ochs et al, 2004) 23/05/2017 23 CVID is thought of as a heterogonous group of disorders with an intrinsic B-cell defect or a Bcell dysfunction which are related to abnormal T-cell - B-cell interactions. In the majority of cases, there is no family history of a related/similar defect, although another family member may have selective IgA deficiency, IgG deficiency, or – rarely – CVID. Although diagnosed in 1953, the fundamental cause(s) of CVID remains unknown. 23/05/2017 24 Due to the unclear genetic nature of CVID, a clear pattern of inheritance has not been defined. In some instances, more than one family member is found to be deficient in one or more types of immunoglobulins. - for example, it is not too unusual for one family member to have CVID whilst another may have selective IgA deficiency. Because of the variability in age at onset and also within the clinical and laboratory findings, as well as the evidence of an unpredictable genetic component, it is thought that CVID is not caused by a single gene defect. 23/05/2017 25 In the past few years, mutations in several different genes have been found to be associated with CVID. These include inducible co-stimulatory (ICOS) in one family and a protein on B-cells (CD19) in several families as causes of autosomal recessive CVID Mutations in a cell receptor (TACI) for two factors (BAFF or APRIL) needed for normal growth and regulation of B-cells have also been found in about 10% of patients with CVID. A causative role of these mutations in the immune defect is not yet clear since some of these mutations can be found in people with normal immunoglobulins. 23/05/2017 26 The main phenotype of CVID is a lack of immune globulin, and hence of antibodies, which occur as a result of a variable block in B-cell differentiation. Most patients with CVID have normal numbers of B-cells (although a few do have low B-cell numbers). In addition, a few males diagnosed with CVID may actually have an atypical XLA, and should be checked for Btk gene mutations. Unlike the mature B-memory cells found in nonantibody deficient people, B-cells found in people with CVID whilst possessing the characteristics of immature B-cell lymphocytes, have deficiencies in actual numbers and the activation of memory Bcells. 23/05/2017 27 Some patients with CVID also have associated T-cell defects. In these patients, although T-cell subsets are generally normal, many of them have lymphopaenia. Then there is a subgroup of CVID patients (2530%) who have increased numbers of CD8+ lymphocytes with normal or decreased numbers of CD4+ lymphocytes, and a reduced CD4/CD8 ratio. Finally, many of these patients have a possible persistence of naϊve T-cells. These findings may be as a result of the T-cells continually being required for chronically infected tissues. 23/05/2017 28 ◦ Other potential problems for patients with CVID include: ◦ Reduced expression of cell surface molecules involved in adhesion (adhesion-switching defects). ◦ Despite the main characteristic of CVID being profound antibody defects, several types of immune over-activation could also contribute to some of the clinical pathology, and hence the signs and symptoms of CVID. ◦ Monocyte/macrophage defects which may cause the immune system to be altered away from antibody production to monocyte production. ◦ This monocyte activation may also be involved in the pathogenesis of the chronic inflammatory and granulomatous complications experienced by some patients with CVID 23/05/2017 29 Some CV ID patients have inflammatory bowel disease (either Crohn’s disease or ulcerative cholitis) presenting with atypical inflammatory disease resulting in diarrhoea, malabsorption & weight loss. Some may also have chronic malabsorption syndrome . Patients are also at risk of GI infections, which may be caused by: ◦ ◦ ◦ ◦ ◦ Giardia lamblia Salmonella Shigella Campylobacter Other odd rarer enteropathogenic organisms 23/05/2017 30 Autoimmune disorders are more frequent in CVID patients (and their relatives) than in the general population. 20-25% of patients with CVID develop one or more autoimmune conditions, such as: ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Rheumatoid arthritis Dermatomyositis Scleroderma SLE Autoimmune haemolytic anaemia Immune thrombocytopaenic purpura Autoimmune neutropaenia Chronic active hepatitis Parotitis Alopaecia Primary billiary cirrhosis Guillon-Barré syndrome 23/05/2017 31 Both adult and paediatric CVID patients may develop noncaseating granulomas of the lung, spleen, liver, skin and other tissues – these resemble sarcoidosis There is also an unusually high incidence of lympho-reticular and gastrointestinal malignancies in older patients with CVID However, as with other congenital immunodeficiencies, the lymphomas in CVID tend to be extranodal in origin and are of Bcell lineage. 23/05/2017 32 Selective Immunoglobulin A Deficiency (SIgA) Selective IgA deficiency is a primary immunodeficiency disease and is the most common of the primary antibody deficiencies. Total immunoglobulin A deficiency (IgA) is defined as an undetectable serum immunoglobulin A (IgA) level at a value < 5 mg/dL (0.05 g/L), whilst partial IgA refers to detectable but decreased IgA levels that are more than 2 standard deviations below normal age-adjusted means. IgA is commonly associated with normal B lymphocytes in peripheral blood, normal CD4+ and CD8+ T cells, and, usually, normal neutrophil and lymphocyte count 23/05/2017 33 Anti-IgA autoantibodies of the IgG and/or IgE isotype may be present. Peripheral blood may also be affected by autoimmune cytopaenias, e.g., autoimmune thrombocytopaenia, and patients may have other autoimmune phenomena. Thus IgA deficiency is a heterogeneous disorder, and the results of intensive study are beginning to identify genetic loci and molecular pathogeneses that contribute to various subtypes of this disorder. Several lines of evidence suggest that, in many cases, IgA and common variable immunodeficiency (CVID) have a common pathogenesis, whilst other data indicate different genetic risk factors. 23/05/2017 34 Family studies show variable inheritance patterns. Familial inheritance of IgA deficiency occurs in approximately 20% of cases, whilst, within a number of families, IgA and CVID are associated. Many IgA deficient patients are asymptomatic and are identified by finding a laboratory abnormality, without any apparent associated clinical disease. Some patients with IgA deficiency may have the following associated conditions: 23/05/2017 35 Deficits in one or more immunoglobulin G (IgG) subclasses (this accounts for 20-30% of IgA-deficient patients, many of whom may have total IgG levels within the normal range) or A deficient antibody response to pneumococcal immunisation (specific polysaccharide antibody deficiency). Some patients with IgA later develop CVID, although family members of patients with CVID may have only selective IgA deficiency. Primary IgA deficiency is permanent, and belownormal levels have been found to remain static and to persist for more than 20 years, although, interestingly, there is one report documents a rare case of reversion (Desar et al, 2007). 23/05/2017 et al. 36 SEVERE COMBINBED IMMUNE DEFICIENCY (SCID) T-CELL/COMBINED IMMUNE DEFICIENCY 23/05/2017 37 ADA SCID Just a (very) brief look at another type of PID ADA SCID is an autosomal recessive disorder ADA = adenosine deaminase (an enzyme of purine metabolism) PNP = purine nucleoside phosphorylase ADA (and PNP) acts sequentially on purine nucleosides - namely adenine and guanine. 23/05/2017 38 ADA is expressed in all cells, but it serves an extremely essential protective role in lymphocytes. Lymphocytes are exceptionally sensitive to metabolites from the breakdown of nuclear acids if ADA is absent. This results particularly in the accumulation of two metabolites - namely : dATP (deoxyadenosine triphosphate) and dGTP (deoxyguanosine triphosphate) These metabolites are toxic to lymphocytes. 23/05/2017 39 OTHER MUTATED GENES CAUSING SCID JAK3 = 19p13.1 (Janus Kinase 3) RAG1 = 11p13 (Recombinase-Activating Gene 1) RAG2 = 11p13 (Recombinase-Activating Gene 2) CD45 = 1q31-q32 X-linked SCID = Xq13.1 23/05/2017 40 ADA SCID MUTATIONS To date, there have been 54 unique mutations identified for this gene, as opposed to only 3 for the CD45 gene and as many as 169 for the IL2RG gene Of these 54 unique mutations, 37 are missense mutations, 9 are splice-site mutations, 4 are frameshift deletions, 3 are nonsense mutations, and 1 is an inframe and gross deletion. (Kalman et al 2004) 23/05/2017 41 SUMMARY These are just a very few of the many, many different types of primary immunodeficiency disorders, and mainly we have looked at just 3 42antibody deficiencies. Antibody deficiencies make up most of the PIDs that nurses will see and deal with, which is why this session concentrated on them. Other PID groups – combined/severe combined immune deficiencies, phagocyte deficiencies and complement deficiencies are also very interesting but time would only allow for SCID to be very briefly discussed. 23/05/2017 42 REFERENCES Desar IM, Weemaes CM, van Deuren M, van der Meer JW. Reversible hypogammaglobulinaemia. Neth J Med. Nov 2007;65(10):381-5. Kalman L, Lindagren ML, Kobrynski L et al. (2004) Mutations in genes for T-cell development: IL7R, CD45, L2RG, JAK3, RAG1, RAG2, AETEMIS and ADA, and severe combined immunodeficiency: HuGE review Genetics in Medicine 6:1 16-26 Ochs HD, Stiehm, ER, Winkelstein JA (2004) Antibody Deficiencies in Stiehm ER, Ochs HD, Winkelstein JA (eds) Immunologic Disorders in Infants and Children (5th. Ed) Philadelphia: Elsevier Saunders 23/05/2017 43 Rotter JI (2004) Approaching the Genetics of Common Diseases PowerPoint Slides; Director of Research and Co-Director, Medical Genetics Institute, Cedars-Sinai, Board of Governors’ Chair in Medical Genetics, Cedars-Sinai, Director, Division of Medical Genetics, CedarsSinai, Professor of Medicine, Pediatrics & Human Genetics, UCLA 23/05/2017 44 FURTHER READING Chapel, H., Haeney, M., Misbah, S., Snowden, N. (2014) Essentials of Clinical Immunology (6th. Ed.) WileyBlackwell, Oxford, UK Male, D., Brostoff, J., Roth, D.B. Roitt, I. (2012) Immunology (8th. Ed.) Elsevier, Saunders, Philadelphia, USA Murphy, K. (2011) Janeway's Immunobiology (8th. Ed.) Garland Science, New York, USA Nairn, R., Helbert, M. (2007) Immunology for Medical Students (2nd. Ed.) Elsevier Mosby, Edinburgh, UK Stiehm, E.R., Ochs, H.D., Winkelstein, J.A. (2004) Immunologic Disorders in Infants and Children (5th. Ed.) Elsevier Saunders, Philadelphia, USA 23/05/2017 45