* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
RNA silencing wikipedia , lookup
Sexual dimorphism wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Point mutation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Designer baby wikipedia , lookup
Non-coding RNA wikipedia , lookup
Gene expression programming wikipedia , lookup
Genome evolution wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Genome (book) wikipedia , lookup
Microevolution wikipedia , lookup
Neocentromere wikipedia , lookup
Y chromosome wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenetics of human development wikipedia , lookup
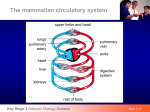
EPIGENETIKA SUMMER 2011 Petr Svoboda mail: tel: [email protected] 241063147 X-INACTIVATION Costs and benefits of sex “The outstanding puzzle of evolutionary biology” Sex is very costly - A population of sexually-reproducing organisms has 50% of fitness (reproductive rate) compared to asexually reproducing population of the same size. Still, the majority of multicellular organisms reproduces sexually. Sexual reproduction must have some compensating advantage - sex can accelerate the rate of evolution (combination of good traits) - sex offers increased variability (gene pools) - genome maintenance (reduction of deletetious mutations) A population of sexually reproducing organisms can, under some conditions, evolve faster than a similar number of asexual organisms. • If favorable mutations arise more frequently, Fisher's argument works: the sexual population evolves faster. Each new favorable mutation will usually arise in an individual that does not already possess other favorable mutations; the greater speed with which the different favorable mutations combine together causes the sexual population to evolve faster. • If favorable mutations are rare, each one will have been fixed in the population before the next one arises. New favorable mutations will always arise in individuals that already carry the previous favorable mutation. Sexual and asexual populations then evolve at the same rate. a.k.a. Fisher’s model Figure: evolution in (a) asexual and (b) sexual populations. The mutations A, B and C are all advantageous. In the asexual population, an AB individual can arise only if the B mutation arises in an individual that already has an A mutation (or vice versa.) In the sexual population, the AB individual can be more easily formed by breeding of a B mutation-bearing individual with an A mutationbearing individual. (c) If favorable mutations are rare, each will have been fixed before the next mutation arises, and sexual populations will not evolve more rapidly. Role of sexual reproduction in complex genome maintenance? BENEFICIAL NEUTRAL DELETERIOUS GENOME NATURAL SELECTION Costs and benefits favourable mutations haploid diploid sexual asexual deleterious mutations parasitic sequences Evolution of sex chromosomes - many different sex-determining systems in plants and animals with separate sexes. - in some species, sex is determined by environmental factors (control expression of genes leading to male or female development) - other species have evolved genetic systems involving specialized sex chromosomes. - sex chromosomes have arisen independently in many animal groups. Also found rarely in plants. It looks like sex chromosomes were once homologs (a pair of equivalent autosomes—the non-sex chromosomes) that evolved different morphology and gene content because they lost their ability to recombine. Suppression of recombination is thought to start around the sex-determining region, but may eventually affect much of the sex chromosomes. In the absence of recombination, the two chromosomes of a pair evolve separately and one of the often deteriorates. Unequal genetic load must be then compensated by some mechanism. Faust’s exercise of making males and females from hermaphrodites start with a hermaphrodite with 4 chromosomal pairs make two sexes with sex chromosomes in a few steps Extreme variability in regulation of sex determination each taxon – different solution >350 MYA MAMMALIA Mus AMPHIBIA Xenopus PISCES Danio Why is it evolving so fast? >400 MYA CHORDATA >600 MYA DEUTEROSTOMES ECHINODERMATA COELOMATES PROTOSTOMES Strongylocentrotus ARTHROPODA Drosophila NEMATODA Caenorhabditis EUMETAZOA PSEUDOCOELOMATES Haplodiploid sex determination Complementary sex determination • ploidy determined by fertilization (no sex chromosomes) • observed by a priest Johann Dzierzon in 1845: virgin queens, which have not taken a mating flight produce only male progeny = the first description of sex determination! • male bee (drone) n=16 chromosomes, develop from unfertilizede ggs • female (worker or queen) 2n=32 chromosomes • about 20% of animals use haplodiploid mode – males are parthenogenotes developing from unfertilized eggs, females develop from fertilized eggs However, there is one problem: Inbreeding studies yielded also diploid males! How do you explain that? Complementary sex determination • complementary sex determination locus model • heterozygosity required for a female (2 different sex determining alleles) NORMAL BREEDING heterozygosity = females female male hemizygosity = males unfertilized eggs fertilized eggs male male female female Complementary sex determination homozygosity is lethal to males because diploid males are eaten by workers soon after they hatch from eggs, leaving empty cells behind INBREEDING female male heterozygosity = females hemizygosity = males homozygosity = males unfertilized eggs fertilized eggs male male male female Is there any molecular evidence for this wild story? • yes, indeed. Complementary sex determiner gene (csd) • csd is a potential splicing factor existing in at least 15 allelic variants • csd inactivation causes switch to males, •csd targets fem gene, fem is spliced differently in female and male cells. http://www.nature.com/scitable/topicpage/sex-determination-in-honeybees-2591764 Sex determination involving sex chromosomes XX/X0 sex determination - females have two copies of the sex chromosome (XX) - males have only one (X0). The 0 denotes the absence of a second sex chromosome. - found in numerous insects (grasshoppers, crickets, and cockroaches) and other invertebrates. - C. elegans: male with one sex chromosome (X0); hermaphrodite with a pair of chromosomes (XX). XX/XY sex chromosomes - females have two of the same kind of sex chromosome (XX) - males have two distinct sex chromosomes (XY). - found in most mammals and insects (Drosophila). - mammals have a SRY gene on the Y chromosome that determines maleness - fruit fly use the presence of two X chromosomes to determine femaleness. ZW sex chromosomes - ZW sex-determination system is reversed compared to the XY system - females have two different kinds of chromosomes (ZW) - males have two of the same kind of chromosomes (ZZ). -found in birds and some insects (Lepidoptera = butterflies) and other organisms. Genes in the ZW region in birds are autosomal in mammals, and vice-versa; therefore, it is theorized that the ZW and XY couples come from different chromosomes of the common ancestor. A paper published in 2004 (Frank Grützner et al, Nature; DOI:10.1038/nature03021) suggests that the two systems may be related. According to the paper, platypuses have a ten-chromosome–based system, where the chromosomes form a multivalent chain in male meiosis, segregating into XXXXX-sperm and YYYYYsperm, with XY-equivalent chromosomes at one end of this chain and the ZW-equivalent chromosomes at the other end. Different strategies to compensate unequal genetic load XX/XY upregulation of expression in males XX/XY silencing of one chromosome in females XX/X0 reducing expression of both chr. in females Straub 2007 Different strategies to compensate unequal genetic load Straub 2007 XX/XO Caenorhabditis elegans Stothard 2003 • reducing expression of both chromosomes in females • X:A ratio determines sex and dosage compensation • dosage compensation complex (DCC) - at least poly10 peptides XX/XO Caenorhabditis elegans Stothard 2003 • condensin complexes function during mitosis and meiosis for DNA compaction and sister chromatid resolution • DCC recruited to specific binding sites on chromosome X • mechanism of the actual 50% down-regulation is not clear. XX/XY Drosophila melanogaster • upregulation of expression in males (increased expression from the X chromosome Lucchesi 2005 • identification of the dosage compensation complex – MSL • dosage compensation involves chromatin modification: H4K16 • H4K16 is unique among acetylation marks. In yeasts, it plays a role in maintaining boundary between silent and active chromatin MSL - male-specific lethal XX/XY Drosophila melanogaster RNA Amrein 2000 HAT activity Homo sapiens/Mus musculus XX/XY - dosage compensation by inactivating one X in female cells 4 steps Counting Choice Initiation Maintenance - if more than one, choose to inactivate, so one remains active - random vs. non-random - initiation and propagation of chromosome-wide silencing - throughout subsequent cell division 2 types of X-inactivation XCI Xi Xa Xic Xce = X chromosome inactivation = inactive X = active X = X inactivation center = X-controlling element (Xist/Tsix) Imprinted X-inactivation - in early embryos, extraembryonic lineage (trophoblast and primitive endoderm) Random X-inactivation -in the epiblast, completed by 5.5.-6.5 dpc Meiotic Sex Chromosome Inactivation Thorvaldsen 2006 X-inactivation and reactivation escape in PGCs MSCI imprinted imprinted imprinted random escape Turner 2007 Meiotic Sex-Chromosome Inactivation PMSC – post-meiotic silencing complex Turner 2007 Meiotic silencing of unsynapsed chomatin Thorvaldsen 2006 X-inactivation and reactivation escape in PGCs imprinted imprinted imprinted random escape X-inactivation during preimplantation development Imprinted X-inactivation - inactive X inherited or de novo silencing after fertilization? - pre-inactivation hypothesis - sex chromosome inactivation during spermatogenesis - XY body in spermatocytes, MSCI (meiotic sex chr. inact.) - MSCI not fully understood, different from XCI (Xist independent, specific chromatin modifications including histone variant H2AX) - staining of 2-cell embryos indicate lack of active transcription on the paternal X - some data support reversion into the active state after meiosis - “de novo” model - Xp active at fertilization, silenced later - staining of 2-cell embryos showing biallelic expression - Xist dependent (Xist expressed at the 2-cell stage) Xist and Tsix Avner 2001 Xist and Tsix http://bioweb.wku.edu/courses/biol566/L9XchromSilencing.html Figure 1. Mouse and Human Xic/XIC and Xist/XIST. A. area surrounding the XIST/Xist gene on human and mouse X-chromosomes. Human domain is inverted from mouse relative to telomere. The identification of a human Tsx homolog is unclear. B. Comparison of the mouse Xist and human XIST genes. * = alternative splicing sites. Mouse has a second promoter that has not been found in other Xist/XIST genes analysed to date. Extensive alternative splicing of the human gene has been described yielding isoforms that lack exon 4, half of exon6, exon7 or include the last two introns. Xist http://bioweb.wku.edu/courses/biol566/L9XchromSilencing.html Longest Xist 17.9 kb. Longest XIST 19.3 kb. Mouse and human Xist/XIST show 49% sequence identity which is lower than 5' & 3' UTR regions but slightly higher than introns. Several short stretches of high homology and six repeated elements A-F. No open reading frame. Must operate as polyadenylated RNA. Xist http://bioweb.wku.edu/courses/biol566/L9XchromSilencing.html http://bioweb.wku.edu/courses/biol566/L9XchromSilencing.html Tsix -two promoters and two polyadenylation sites. no significant open reading frames. - Tsix transcripts of up to 4 kb can be produced by splicing. - Tsix is not the counting element (Males lacking Tsix do not inactivate). - Tsix RNA is antisense to Xist and reduces its steady-state level while subsequently promotes Xa choice by increasing the affinity of the cis-linked counting element for blocking factor. - the spliced form of Tsix RNA contains only 2 kb of overlap with the mature Xist transcript. This overlap occurs within a domain of Xist that is critical for silencing activity - antisense transcription throught the Xist sequence is important (Deletion mutants lacking the overlap) Avner 2001 Tsix X-inactivation during preimplantation development Imprinted X-inactivation - paternal Xist expression activated at the 2-cell stage - Xist silencing of the maternal X (Xm) is unclear - Tsix (maternal) is detected first at the 8-cell stage - Xist accumulates on the Xp (initiation event) from the 4-cell on - initial chromatin changes found during the 8-32-cell stages - hypoacetylation H3K9 - hypomethylation H3K4 - EED/EZH2 enrichment mediates H3K27 methylation on the Xi - initiation vs. maintenance changes unknown - gradient of silencing from the Xic suggests that silencing is progressive, mediated by Xist RNA spreading - ICM cells reverse imprinted XCI, trophectoderm cells maintain it X-inactivation during preimplantation development Random X-inactivation - paternal Xist silencing reversed after early blastocyst - Xist dispersed or absent - no EED/EZH2 association, - random X-inactivation initiates during implantation and is complete around day 6.5 dpc - initiation is characterized by downregulation of Tsix and upregulation of Xist - Xist coats the Xi in cis - chromatin modifications - DNA methylation is a late step - once established, the Xi is clonally propagated such that females are functionally mosaic for X-linked traits. - epigenetic modification can later maintain Xi repression in a Xistindependent manner Thorvaldsen 2006 Xist and Tsix XCI Xi Xa Xic Xce = X chromosome inactivation = inactive X = active X = X inactivation center = X-controlling element (Xist/Tsix) Avner 2001 doesn’t seem to be the case http://bioweb.wku.edu/courses/biol566/L9XchromSilencing.html XCI in differentiating female ES cells MacroH2A is recruited to the Xi by Xist. followed by exclusion of H2ABbd variant from Xi. (formation of Barr body). Xist/XIST espression is not necessary to continue Xi after establishment. DNA methylation appears to be extremely important for the stability and maintenance of gene silencing on Xi. DNA methylation concerns promoter regions, overall is the inactive X hypomethylated! http://bioweb.wku.edu/courses/biol566/L9XchromSilencing.html XCI in differentiating female ES cells Turner syndrome - 45, X or 46, X, abn X • fairly common (10% od spontaneous abortions) • 1 of 40 develops to birth, then the phenotypic effects are relatively mild because each cell has a single functioning X chromosome like those of XX females. • phenotypic female with gonadal dysgenesis and sexual immaturity, have primary amenorrhea (failure to menstruate), infertility, short stature, webbed neck, increased carrying angle at the elbow, cardiovascular and renal abnormalities • 45,X in more than half the patients Number of Barr bodies = zero. Incidence: 1 of 2500 female births Why does Turner syndrome occur at all, since only one X chromosome is normally active? There are two active X chromosomes during ovarian development, and certain genes appear to need to be active for normal ovarian function. Turner syndrome oocytes virtually gone by the age of 2 years Klinefelter syndrome - 47, XXY(48, XXXY) • males (Y chromosome). • the phenotypic effects of the extra X chromosomes are mild because, the extra Xs are inactivated and converted into Barr bodies • male with small testes, hyalinized testicular tubules, and azoospermia (failure to produce normal amounts of sperm), resulting in infertility and variable signs of hypogonadism, social pathologies, somewhat reduced IQ, postpubertal testicular failure • may have additional X chromosomes, if so, more likely to be mentally retarded • demonstration in humans that sex is determined by presence or absence of Y chromosome, rather than number of X chromosomes Number of Barr bodies = extra X’s inactivated Incidence: 1 of 1000 male births XYY syndrome - found as 47,XYY, or 48,XXYY 47,XYY - occurs 1/1000 in male live births - occurs 4-20 per 1000 inmates 48,XXYY - incidence 1/20-40 000 - 50 times higher in prison inmates than in newborn population XX males - incidence 1 in 20,000 - have X-Y interchange - Sry transgenic mice, XX become male XXX, XXXX, XXXXX females - mild phenotypic effects because in each cell all the extra X chromosomes are inactivated. - number of Barr bodies = number of X chromosomes minus one. IMPRINTING Discovery of Imprinted Genes • experimental manipulation of mouse embryos in the early 1980's showed that normal development requires the contribution of both the maternal and paternal genomes. • gynogenetic embryos (two female genomes) show relatively normal embryonic development, but poor placental development. • androgenetic embryos (two male genomes) show very poor embryonic development but normal placental development. • it is now known that there are around 100 imprinted genes in humans and mice, many of which are involved in embryonic and placental growth and development • the gynogenetic embryos have twice the normal level of maternally expressed genes, and completely lack expression of paternally expressed genes, whereas the reverse is true for androgenetic embryos. • no naturally cases of parthenogenesis exist in mammals (Jesus does’nt count!) • manipulation of a paternal methylation imprint controlling the Igf2 locus allowed the creation of rare individual mice with two maternal sets of chromosomes (not a true parthenogenote). http://atlasgeneticsoncology.org/Deep/GenomImprintID20032.html Mouse germ cell pronuclear transplant experiments convincingly demonstrate a different agenda for sperm- versus egg-derived nuclear genomes during development. Development in the absence of a sperm-derived genome (middle column) shows fairly good development of the embryo proper but failed development of the trophoblast lineage. Development in the absence of an eggderived genome (right column) shows failed development of the embryo proper but exuberant trophoblast growth. Imprinting is a cause of phenotypes in uniparental disomies • In 1980 Engel introduced the concept of uniparental disomy (UPD). • Uniparental disomy (UPD) arises when an individual inherits two copies of a chromosome pair from one parent and no copy from the other parent. • In the rare circumstance of UPD a baby may have two copies of one of his/ her mother’s chromosome and no copies of that chromosome from his/ her father. This is called maternal UPD. Paternal UPD is when a child inherits two copies of a specific chromosome from his/ her father and no copies of that chromosome from his/ her mother. •This abnormality in inheritance may lead to health concerns in a child. • UPD can result in rare recessive disorders, or developmental problems due to the effects of imprinting. UPD may also occur with no apparent impact on the health and development of and individual. Prader-Willi syndrome, Angelman syndrome, Beckwith-Wiedemann syndrome Described in detail in Buiting 2010 Described in detail in Choufani 2010 http://www.mgu.har.mrc.ac.uk/research/imprinting/imprin-viewmaps.html Maps of imprinted genes General features of imprinted genes • typically clustered, clusters may contain monoallelic expression of genes from each parent. • clusters contain imprint control regions and a non-coding RNA is often found associated with it (H19, Air …) • ICRs show parent-of-origin dependent epigenetic modifications (methylation) • many related to growth control (battle of the sexes hypothesis) • it has been reported that imprinted genes tend to have smaller introns. • some genes imprinted only in neural tissues Robertson 2005 Mouse distal 7 imprinted region H19 - a noncoding RNA!! CTCF http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=604167 • CCCTC binding factor with 11 zinc fingers • highly conserved in vertebrates (93% identity human-avian) • binds to regulatory sequences in numerous loci, including H19/IGF2 • binding is methylation sensitive, protects DNA from methylation • insulator - regulates access of enhancers/separates functional domains • boundary element - blocks spread of heterochromatin Fedoriw 2004 Binding of CTCF is essential for proper H19/IGF2 imprinting H19 is a non-coding RNA • ~2.3 kb long, maternally expressed • integrity of elements controlling H19 transcription essential for Igf2 imprinting • H19 RNA is not essential for Igf2 imprinting • ectopic H19 overexpression can affect viability but targeted deletion makes no obvious phenotype (but expression of other is genes affected) • H19 could be a primary miRNA precursor For recent data se Gabory et al., Bioessays 2010 Reciprocal imprinting, ICR and non-coding RNAs is a common theme Lucifero 2004 Variable timing of maternal imprinted marks De novo DNMT3a/b, DNMT3L Two modes of imprinting Insulator model ncRNA model Air ncRNA expression involves in silencing of upstrean genes IMPRINTING DEFECTS IN ARTs X-inactivation and imprinting evolution Pauler 2007