* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ppt
Survey
Document related concepts
Oxidative phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Butyric acid wikipedia , lookup
Genetic code wikipedia , lookup
Citric acid cycle wikipedia , lookup
Proteolysis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Transcript
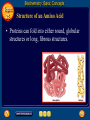
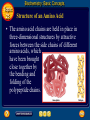
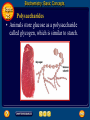
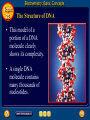
Topic 25 Table of Contents Topic 25 Topic 25: Biochemistry Basic Concepts Additional Concepts Biochemistry: Basic Concepts Topic 25 Molecules of Life • Many of the most important molecules in your body are polymers. • Proteins, carbohydrates, and nucleic acids, all extremely large molecules, are formed from small monomer subunits. • Although lipids are usually not considered to be polymers, they, too, are formed from smaller molecules that have been linked together. Biochemistry: Basic Concepts Topic 25 Molecules of Life • You need relatively large amounts of proteins, carbohydrates, and lipids in your diet. • Complex reactions in your cells use some of these molecules and a few others to make a fourth group of biomolecules, the nucleic acids. Biochemistry: Basic Concepts Topic 25 Biochemistry • The study of the chemistry of living things is called biochemistry. • This science explores the substances involved in life processes and the reactions they undergo. • Other than water, which can account for 80 percent or more of the weight of an organism, most of the molecules of life— the biomolecules—are organic. Biochemistry: Basic Concepts Topic 25 Biochemistry • The elemental composition of living things is different from the relative abundance of elements in Earth’s crust. • Oxygen, silicon, aluminum, and iron are the most abundant atoms in Earth’s crust. Biochemistry: Basic Concepts Topic 25 Biochemistry • However, more than 95 percent of the atoms in your body are hydrogen, oxygen, carbon, and nitrogen. • All four of these elements can form the strong covalent bonds found in organic molecules. Biochemistry: Basic Concepts Topic 25 Biochemistry • Along with two other elements, sulfur and phosphorus, they are the only elements needed to make most of the proteins, carbohydrates, lipids, and nucleic acids found in every cell. Biochemistry: Basic Concepts Topic 25 Proteins • A protein is an organic polymer composed of amino acids bonded together in one or more chains. • An amino acid has a central carbon atom, to which are bonded a carboxyl group, an amino group, a hydrogen atom, and a variable side chain designated as R, as shown in the following structural formula. Biochemistry: Basic Concepts Topic 25 Structure of an Amino Acid Biochemistry: Basic Concepts Topic 25 Structure of an Amino Acid • Amino acids bond to each other by forming a peptide bond, an amide group formed by a condensation reaction between the carboxyl group of one amino acid and the amino group of another. Biochemistry: Basic Concepts Topic 25 Structure of an Amino Acid • Two amino acids linked by a peptide bond form a dipeptide. Biochemistry: Basic Concepts Topic 25 Structure of an Amino Acid • A chain of two or more amino acids linked by peptide bonds is called a peptide. • The term polypeptide is applied to a chain of ten or more amino acids. • Proteins may have one or several polypeptide chains, and each chain must have an exact sequence of amino acids. Biochemistry: Basic Concepts Topic 25 Structure of an Amino Acid • Proteins can fold into either round, globular structures or long, fibrous structures. Biochemistry: Basic Concepts Topic 25 Structure of an Amino Acid • The amino acid chains are held in place in three-dimensional structures by attractive forces between the side chains of different amino acids, which have been brought close together by the bending and folding of the polypeptide chains. Biochemistry: Basic Concepts Topic 25 Enzymes • Many of the proteins in an organism act as enzymes. These proteins catalyze chemical reactions—speeding up reactions or allowing the reactions to take place at a low temperature. Biochemistry: Basic Concepts Topic 25 Enzymes • The reactants in an enzyme-catalyzed process are called substrates. The substrate(s) bind to the enzyme at a location called the enzyme’s active site, forming an enzyme-substrate complex. • This interaction enables the substrate(s) to react with a much lower activation energy than they would without an enzyme. Biochemistry: Basic Concepts Topic 25 Enzymes Click box to view movie clip. Biochemistry: Basic Concepts Topic 25 Enzyme Action • Substrates are brought close together in the active sites of an enzyme, which lowers the activation energy of the reaction by facilitating the bonding together of the substrates to form a product. Biochemistry: Basic Concepts Topic 25 Enzyme Action • After the substrates have reacted, the product is released. • The enzyme is then able to bind more substrate molecules and continue catalyzing the reaction. Biochemistry: Basic Concepts Topic 25 Carbohydrates • Familiar carbohydrates include sugars, starches, and cellulose. • Simple carbohydrates consist of a chain of carbon atoms having hydroxyl (–OH) groups and a carbonyl group, often in the form of an aldehyde group. Biochemistry: Basic Concepts Topic 25 Monosaccharides • The simplest carbohydrates are the simple sugars, or monosaccharides, which commonly have five or six carbon atoms. • Glucose, the main ingredient in corn syrup, is a familiar monosaccharide. Biochemistry: Basic Concepts Topic 25 Monosaccharides • Glucose has the molecular formula C6H12O6 and can be represented by the following structures. Biochemistry: Basic Concepts Topic 25 Monosaccharides • The most common simple sugars are glucose, fructose, and ribose. Biochemistry: Basic Concepts Topic 25 Polysaccharides • A polymer of many monosaccharides bonded into a chain is called a polysaccharide. • Starch is a polysaccharide that consists only of glucose units. Biochemistry: Basic Concepts Topic 25 Polysaccharides • Plants also link glucose units together in a different way to form the polysaccharide cellulose, which forms plant cell walls. Biochemistry: Basic Concepts Topic 25 Polysaccharides • Animals store glucose as a polysaccharide called glycogen, which is similar to starch. Biochemistry: Basic Concepts Topic 25 Lipids • Lipids are the nonpolar substances—fats, waxes, and oils—produced by living things. • Lipids are not polymers, and their chemical structures vary widely. Click box to view movie clip. Biochemistry: Basic Concepts Topic 25 Fatty Acids • The most familiar lipids are the plant oils and animal fats. • These lipids are esters of fatty acids, which are carboxylic acids with long, straight hydrocarbon chains usually having between 12 and 24 carbon atoms. Biochemistry: Basic Concepts Topic 25 Saturated Fatty Acid • The simplest fatty acids are the saturated fatty acids, which have no double bonds between carbon atoms. • Stearic acid is found in pork and beef tissue. Biochemistry: Basic Concepts Topic 25 Monounsaturated Fatty Acid • Many other fatty acids have one or more double bonds between carbon atoms and, as a result, are unsaturated fatty acids. • Oleic acid is a major component of olive oil. Biochemistry: Basic Concepts Topic 25 Triglycerides • Animal fats and plant oils are made up primarily of triglycerides, molecules in which three fatty acids are bonded to a glycerol molecule by ester linkages, as shown in the following diagram. Biochemistry: Basic Concepts Topic 25 Triglycerides • Phospholipids are triglycerides in which a polar phosphate group, instead of a third fatty acid, is bonded to the glycerol. • Cell membranes consist of a double layer of phospholipid molecules. Biochemistry: Basic Concepts Topic 25 Triglycerides • The membranes of living cells are formed by a double layer of lipids called a bilayer. Biochemistry: Basic Concepts Topic 25 Other Lipids • Another class of lipids, steroids, consists of compounds whose basic structure is very different from those of other lipids, as shown below. • Cholesterol, vitamin D, and some hormones are steroids. Biochemistry: Basic Concepts Topic 25 The Functions of Lipids • Lipids have two major biochemical roles in the body. • When an organism takes in and processes more food than it needs, excess energy is produced. • The organism stores this excess energy for future use by using it to bond atoms together in lipid molecules. Biochemistry: Basic Concepts Topic 25 The Functions of Lipids • Later, when energy is needed, enzymes break these same bonds, releasing the energy used to form them. • You have learned that carbohydrates also store energy; however, the process is not as efficient as in lipids. • Therefore, long-term storage of energy is usually in the form of lipids. Biochemistry: Basic Concepts Topic 25 Nucleic Acids • The sequence of amino acids in a protein is determined by the genetic information coded into long-chain polymers called nucleic acids. • The monomers that make up nucleic acids are called nucleotides. Biochemistry: Basic Concepts Topic 25 Nucleic Acids • Each nucleotide is made up of three parts: a phosphate group, a five-carbon sugar, and a nitrogen-containing cyclic compound called a nitrogen base. • The structure of a nucleotide is shown. Biochemistry: Basic Concepts Topic 25 Nucleic Acids Biochemistry: Basic Concepts Topic 25 Nucleic Acids Biochemistry: Basic Concepts Topic 25 Nucleic Acids • The common nucleic acids are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). • These names reflect the fact that DNA contains the sugar deoxyribose and RNA contains the sugar ribose. • DNA exists as a pair of polymer chains in which the backbone of each chain consists of alternating phosphate and deoxyribose units. The bases stick out from the backbone. Biochemistry: Basic Concepts Topic 25 The Structure of DNA • This model of a portion of a DNA molecule clearly shows its complexity. • A single DNA molecule contains many thousands of nucleotides. Biochemistry: Basic Concepts Topic 25 Base Pairing • The two chains of DNA are held together because the nitrogen bases of one chain are hydrogen-bonded to the nitrogen bases of the other chain. • Because of the change in angle from one nucleotide to the next, the chains wind into a spiral called a double helix. Biochemistry: Basic Concepts Topic 25 Base Pairing • Four different nitrogen bases are found in DNA: adenine, guanine, cytosine, and thymine. • Adenine hydrogen bonds to thymine, and guanine hydrogen bonds to cytosine. Biochemistry: Basic Concepts Topic 25 Base Pairing • The order of these four nitrogen bases along one of the DNA chains provides the information for the sequences of amino acids in proteins. • Cell mechanisms “read” the DNA sequence in groups of three bases called triplets. • Each triplet codes for a specific amino acid or tells the cell to start or stop making a protein. Basic Assessment Questions Topic 25 Question 1 Label the amino group and the carboxyl group of the dipeptide. Basic Assessment Questions Topic 25 Answer Basic Assessment Questions Topic 25 Question 2 Draw an arrow pointing to the peptide bond. Basic Assessment Questions Topic 25 Answer Basic Assessment Questions Topic 25 Question 3 Draw a square around each variable side chain. Basic Assessment Questions Topic 25 Answer Basic Assessment Questions Topic 25 Question 4 Glucose is a(n) a. polysaccharide. b. amino acid. c. part of cellulose. d. 5-carbon sugar. Basic Assessment Questions Topic 25 Answer The answer is C, part of cellulose. Biochemistry: Additional Concepts Topic 25 Additional Concepts Biochemistry: Additional Concepts Topic 25 Anabolism and Catabolism • The set of reactions carried out by an organism is its metabolism. • Living organisms must accomplish two major functions in order to survive. • They have to extract energy from nutrients in forms that they can use immediately as well as store for future use. Biochemistry: Additional Concepts Topic 25 Anabolism and Catabolism • In addition, they have to use nutrients to make building blocks for synthesizing all of the molecules needed to carry out their life functions. Biochemistry: Additional Concepts Topic 25 Anabolism and Catabolism • A large number of different metabolic reactions take place in living cells. • Some involve breaking down nutrients to extract energy; these are catabolic processes. Biochemistry: Additional Concepts Topic 25 Anabolism and Catabolism • Others involve using energy to build large biological molecules; these reactions are anabolic processes. Biochemistry: Additional Concepts Topic 25 Anabolism and Catabolism • The term catabolism refers to the metabolic reactions that break down complex biological molecules such as proteins, polysaccharides, triglycerides, and nucleic acids for the purposes of forming smaller building blocks and extracting energy. • The term anabolism refers to the metabolic reactions that use energy and small building blocks to synthesize the complex molecules needed by an organism. Biochemistry: Additional Concepts Topic 25 ATP • Catabolism and anabolism are linked by common building blocks that catabolic reactions produce and anabolic reactions use. • A common form of potential chemical energy also links the two processes. Biochemistry: Additional Concepts Topic 25 ATP • ATP (adenosine triphosphate) is a nucleotide that functions as the universal energy-storage molecule in living cells. • During catabolic reactions, cells harness the chemical energy of foods and store it in the bonds of ATP. Biochemistry: Additional Concepts Topic 25 Photosynthesis • What is the source of the energy that fuels metabolism? For most living things, certain wavelengths of sunlight provide all of this energy. • Some bacteria and the cells of all plants and algae, including the brown algae, are able to capture light energy and convert some of it to chemical energy. Biochemistry: Additional Concepts Topic 25 Photosynthesis • Animals can’t capture light energy, so they get energy by eating plants or by eating other animals that eat plants. • The process that converts energy from sunlight to chemical energy in the bonds of carbohydrates is called photosynthesis. Biochemistry: Additional Concepts Topic 25 Photosynthesis • ATP is a nucleotide that contains an adenine nitrogen base, a ribose sugar, and three phosphate groups. Biochemistry: Additional Concepts Topic 25 Photosynthesis • When the final phosphate group is removed from ATP, as modeled by the red dotted line, ADP is formed and energy is released. Biochemistry: Additional Concepts Topic 25 Photosynthesis • During the complex process of photosynthesis, carbon dioxide and water provide the carbon, hydrogen, and oxygen atoms that make up carbohydrates and oxygen gas, which also is formed. • The following net reaction takes place during photosynthesis. Biochemistry: Additional Concepts Topic 25 Photosynthesis • Photosynthesis results in the reduction of the carbon atoms in carbon dioxide as glucose is formed. • During this redox process, oxygen atoms in water are oxidized to oxygen gas. Biochemistry: Additional Concepts Topic 25 Cellular Respiration • Most organisms need oxygen to live. Oxygen that is produced during photosynthesis is used by living things during cellular respiration, the process in which glucose is broken down to form carbon dioxide, water, and large amounts of energy. • Cellular respiration is a redox process; the carbon atoms in glucose are oxidized while oxygen atoms in oxygen gas are reduced to the oxygen in water. Biochemistry: Additional Concepts Topic 25 Cellular Respiration • The net reaction that takes place during cellular respiration is • Note that the net equation for cellular respiration is the reverse of the net equation for photosynthesis. • These two processes complement each other in nature. Biochemistry: Additional Concepts Topic 25 Fermentation • During cellular respiration, glucose is completely oxidized, and oxygen gas is required to act as the oxidizing agent. • Can cells extract energy from glucose in the absence of oxygen? • Yes, but not nearly as efficiently. Without oxygen, only a fraction of the chemical energy of glucose can be released. Biochemistry: Additional Concepts Topic 25 Fermentation • Whereas cellular respiration produces 38 moles of ATP for every mole of glucose catabolized in the presence of oxygen, only two moles of ATP are produced per mole of glucose that is catabolized in the absence of oxygen. Biochemistry: Additional Concepts Topic 25 • • • • Fermentation This provides enough energy for oxygendeprived cells so that they don’t die. The process in which glucose is broken down in the absence of oxygen is known as fermentation. There are two common kinds of fermentation. In one, ethanol and carbon dioxide are produced. In the other, lactic acid is produced. Biochemistry: Additional Concepts Topic 25 Alcoholic fermentation • Yeast and some bacteria can ferment glucose to produce the alcohol ethanol. Biochemistry: Additional Concepts Topic 25 Alcoholic fermentation Biochemistry: Additional Concepts Topic 25 Alcoholic fermentation • Alcoholic fermentation is needed to make bread dough rise, form tofu from soybeans, and produce the ethanol in alcoholic beverages. • Another use of the ethanol that is produced by yeast is as an additive to gasoline, called gasohol. Biochemistry: Additional Concepts Topic 25 Lactic acid fermentation • During strenuous activity, muscle cells often use oxygen faster than it can be supplied by the blood. • When the supply of oxygen is depleted, cellular respiration stops. Biochemistry: Additional Concepts Topic 25 Lactic acid fermentation • Although animal cells can’t undergo alcoholic fermentation, they can produce lactic acid and a small amount of energy from glucose through lactic acid fermentation. Biochemistry: Additional Concepts Topic 25 Lactic acid fermentation Biochemistry: Additional Concepts Topic 25 Lactic acid fermentation • The lactic acid that is produced is moved from the muscles through the blood to the liver. • There, it is converted back into glucose that can be used in catabolic processes to yield more energy once oxygen becomes available. Biochemistry: Additional Concepts Topic 25 Lactic acid fermentation • However, if lactic acid builds up in muscle cells at a faster rate than the blood can remove it, muscle fatigue results. • Buildup of lactic acid is what causes a burning pain in the muscle during strenuous exercise. Additional Assessment Questions Topic 25 Question 1 What effect do enzymes have on the chemical reactions that take place in living things? Additional Assessment Questions Topic 25 Answer speed reactions by lowering activation energy Additional Assessment Questions Topic 25 Question 2 Your cells carry out cellular respiration. What is the function of this process? Additional Assessment Questions Topic 25 Answer releases energy for life processes Additional Assessment Questions Topic 25 Question 3 What process is the reverse of cellular respiration? Additional Assessment Questions Topic 25 Answer photosynthesis Help To advance to the next item or next page click on any of the following keys: mouse, space bar, enter, down or forward arrow. Click on this icon to return to the table of contents Click on this icon to return to the previous slide Click on this icon to move to the next slide Click on this icon to open the resources file. Click on this icon to go to the end of the presentation. End of Topic Summary File