* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ENZYMES (Basic Concepts and Kinetics) (Chapter 8)
Multi-state modeling of biomolecules wikipedia , lookup
Magnesium in biology wikipedia , lookup
Citric acid cycle wikipedia , lookup
Lipid signaling wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Ultrasensitivity wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Biochemistry wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Proteolysis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Restriction enzyme wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Catalytic triad wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
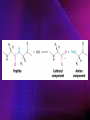
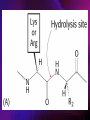
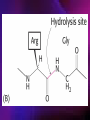
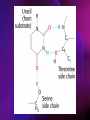
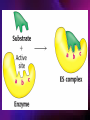
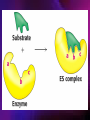
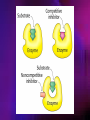
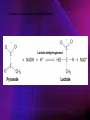
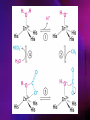
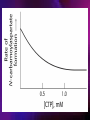
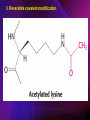
ENZYMES ENZYMES •are biological catalyst •are mostly proteinaceous in nature, but RNA was an early biocatalyst •are powerful and highly specific catalysts carbonic anhydrase Many enzymes require co-factor for activity Apoenzyme + co-factor = holoenzyme NOMENCLATURE: 1. Common name- e.g. trypsin, pepsin 2. Hybrid name- e.g. sucrase 3. Systematic name: EC 2.7.4.4 example: nucleoside monophosphate kinase ATP + NMP ADP + NDP ENZYMES accelerate reactions by facilitating the formation of the transition state Active site • is the region where the substrate binds • contains residue that directly participate in making or breaking of bonds (formation of transition state) • is the region where activation energy is lowered Common features 1. Active site is a three dimensional cleft 2. Takes up a small part of the total volume of an enzyme 3. Are clefts or crevice 4. Substrates are bound to enzymes by multiple weak interactions Two models of the Active site 1.Lock and key 2.Induced-fit Kinetic Properties of Enzymes Michaelis-Menten Equation Factors Affecting Enzyme Activity 1. Temperature 2. pH 3. [S] 4. Presence of Inhibitors TEMPERATURE •As the temperature rises, molecular motion - and hence collisions between enzyme and substrate - speed up. But as enzymes are proteins, there is an upper limit beyond which the enzyme becomes denatured and ineffective. pH •The conformation of a protein is influenced by pH and as enzyme activity is crucially dependent on its conformation, its activity is likewise affected. Kinetic Theory of EnzymeCatalyzed Reaction 1. Effect of [E] involves the reversible formation of an enzyme-substrate complex, which then break down to form one or more products if [S] is constant, v is proportional to [E] 2. Effect of [S] has profound effect on the rate of enzymecatalyzed reaction o At low [S], rate of reaction is 1 order, v is directly proportional to [S] At mid [S], rate of reaction is mixed order proportionality is changing At high [S], rate of reaction is zero order Michaelis-Menten Equation Significance of KM When V= ½ Vmax, what is [S]? The KM of an enzyme is the substrate concentration at which the reaction occurs at half of the maximum rate. There are limitations in the quantitative (i.e. numerical) interpretation of this type of graph, known as a Michaelis plot. The Vmax is never really reached and therefore Vmax and hence KM values calculated from this graph are somewhat approximate. Lineweaver- Burk plot The Effects of Enzyme Inhibitors 1. Competitive In the presence of a competitive inhibitor, it takes a higher substrate concentration to achieve the same velocities that were reached in its absence. So while Vmax can still be reached if sufficient substrate is available, one-half Vmax requires a higher [S] than before and thus Km is larger 2. Non-Competitive With noncompetitive inhibition, enzyme molecules that have been bound by the inhibitor are taken out of the game so • enzyme rate (velocity) is reduced for all values of [S], including • Vmax and one-half Vmax but • Km remains unchanged because the active site of those enzyme molecules that have not been inhibited is unchanged. Most Biochemical Reactions Include Multiple Substrates: A+BP+Q 2 Classes of Multiple Substrate Reactions: 1. Sequential Displacement 2. Double Displacement Sequential Displacement: All substrates bind to the enzyme before any product is release Types • Ordered • Random Example of a sequential ordered mechanism Lactate dehydrogenase The enzyme exist as a ternary complex Example of a Random Sequential Mechanism Creatine kinase Double Displacement (Ping-Pong) Reactions -one or more products are released before all substrates bind the the enzyme - a substituted enzyme intermediate exist Aspartate amino transferase Enzymes employ strategies to catalyze specific reactions 1.Covalent Catalysis- the active site contains a reactive group 2.General Acid base catalysis 3.Metal ion catalysis 4.Catalysis by approximation 1. Covalent Catalysis Example: Chymotrypsin A. Acylation to form the acyl-enzyme intermediate B. Deacylation to regenerate the free enzyme 2. General Acid base catalysis 3. Metal ion catalysis- e.g. carbonic anhydrase Regulatory Strategies: 1. Allosteric Enzyme- e.g. ATCase 2. Multiple of Enzymes: Isoezymes or Isozymes- are homologous enzymes within a single organism that catalyze the same reaction but differ slightly in structure and in Vmax and Km e.g. Lactate dehydrogenase (LDH)- 2 isozmic chains in humans, H (heart) and M (muscles) 3. Reversible covalent modification 4. Proteolytic activation- involves synthesis of enzymes in the ZYMOGEN form Examples: 1. Digestive enzymes 2. Blood clottingcascade of zymogen activations