* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Fatty acid metabolism wikipedia , lookup
Development of analogs of thalidomide wikipedia , lookup
Peptide synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Genetic code wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Transcript
MEDICINAL CHEMISTRYIII
Lecture 2
Wed. 16/ 5/ 1432H
Prof. Dr. Wafaa Zaghary
III-Anthranilates
Flufenamic acid
2—{[3-(Trifluoromethyl)phenyl]amino}benzoic acid.
Uses anti-inflammatory
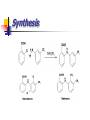
Synthesis
Structure Activity relationships
Substitution on the anthranilic acid ring reduce
activity.
The most active anthranilic acid derivatives have
substituents at position 2`,3` and 6`of the ring
attached to the anthranilic acid nitrogen.
Replacing the –NH- function in fenamic acids
produces less active compounds. Thus ether,
ketones, and thioether are essentially inactive.
The carboxylic function is required at the 2-position
for biological activity.
Isomeric 3-carboxy or 4-carboxy derivatives are
inactive.
A tetrazole function can substitute for carboxylic acid
function with retention of anti-inflammatory activity.
On the basis of structure-activity relationships for
indomethacin and other NSAIDs, and antiinflammatory receptor site consisting of two non
coplaner hydrophobic regions and a cationic centre.
The anti-inflammatory receptor consists of a
largely flat area, a trough to accommodate an
out-of-plane group (such as an aryl ring
acting possibly by charge–transfer type of
interaction) and a cationic site to
accommodate an acidic anion.
IV-Aryl acetic Acids:
Diclofenac Voltaren®
2-[(2,6-dichlorophenyl)amino]benzene acetic.
It is a NSAIDs with balanced cox-1 and cox-2
inhibiting effect.
Indomethacin
It is still one of the most potent NSAIDs in use,
possessing approximately 25 times the activity of
phenylbutazone.
It is more potent than aspirin and acetaminophen.
CN: 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1Hindol-3-acetic acid.
Structure activity relationships
All agents posses a centre of activity can be
represented by carboxylic acid function, enolic function
hydroxamic acid function a sulfonamide or tetrazole.
The activity of ester and amide derivatives of
carboxylic acid is attributed to the metabolic hydrolysis
product.
The center of acidity is located on carbon atom
adjacent to a flat surface represented by aromatic or
heteroaromatic.
Increasing the distance to two to three carbons
diminish activity.
Derivatives of aryl or heteroaryl acetic or propionic
acids are most common.
Substitution of methyl group on the carbon atom
separating the acid centre from the aromatic ring
increase the anti-inflammatory activity.
Group larger than methyl decrease activity.
A second area of lipophilicity which is generally
noncoplaner with aromatic or heteroaromatic ring
enhance activity. The lipophilic function may consist
of an addition aromatic ring or alkyl group.
V-Aryl propionic Acids
Ibuprofen Brufen®
2-(4-isobutylphenyl)propionic acid
It is a NSAID used for the treatment of
mild to moderate pain.
VI-Pyrazolinone derivatives
Metamizole Novalgin®, Baralgin®
It has strong analgesic spasmolytic and
antipyretic but no anti-inflammatory
properties.
VII-Acidic enolic compounds
1.
Pyrazolidine 3,5-diones
Oxyphenbutazone
Anti-inflammatory
Tanderil®
2.
Aryl sulfonamides (oxicames):
Piroxicam
Anti-inflammatory