* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry - Holding
Survey
Document related concepts
DNA-encoded chemical library wikipedia , lookup
Photosynthesis wikipedia , lookup
Chemical biology wikipedia , lookup
Genetic code wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
History of molecular biology wikipedia , lookup
Expanded genetic code wikipedia , lookup
Biomolecular engineering wikipedia , lookup
List of types of proteins wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Carbohydrate wikipedia , lookup
Hypothetical types of biochemistry wikipedia , lookup
Abiogenesis wikipedia , lookup
Transcript
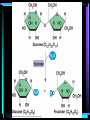
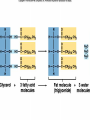
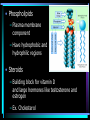
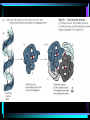
Organic Chemistry Organic compounds contain the element carbon Occur naturally only in living organisms or in their products Out of the 92 elements found in nature, only a few are in organic compounds • Inorganic compounds do not contain carbon – Exception – carbon dioxide (CO2) and other carbonate compounds (CO3) – Examples – water, salts, acids, bases • Living organisms contain both organic and inorganic compounds – Water is the most important inorganic compound Macromolecules • Carbon atoms can be joined covalent into long chains – Straight chained molecules – Branched molecules – Ring molecules • Most cells store small carbon compounds which serve as building blocks for larger molecules • Macromolecules – large molecules formed from smaller organic molecules bonded together – Four major categories: • Carbohydrates • Lipids • Proteins • Nucleic Acids • Polymer – molecules made from repeating units of identical compounds (monomers) which are bonded together Carbohydrates • Composed of: – Carbon – Hydrogen – Oxygen • Ratio of hydrogen to oxygen for each carbon atom (2 H : 1 O) – same as water • General formula – (CH2O)n – N represents the number of repeating units in the chain • Carbohydrate function – Energy source – Cellular structural support • Ex. Cellulose in plant walls • Ex. Chitin in exoskeletons of insects • Simple sugars – Have 3-7 repeating units – Also known as a monosaccharide – Chemical names ALWAYS end in -ose – Ex. Glucose (C6H12O6) – essential energy source for organisms • Disaccharide – Two simple sugars linked together – Ex. Sucrose (table sugar) – additional energy source for organisms – Ex. Lactose – component of milk • Dehydration Synthesis – sugar molecules are bonded together by removing water – Brought on in living cells by the action of enzymes – Important in forming the complex organic compounds an organism needs • Polysaccharide – Three or more simple sugars linked together (polymer) – Ex. Glycogen – molecule form for storage of glucose found in liver and skeletal muscle • Hydrolysis – Breakdown of disaccharides or polysaccharides to yield simple sugars Lipids • Composed of: – Carbon – Hydrogen – Oxygen (less than found in carbohydrates) • Triglycerides: – Fats • Solid at room temperature – Oils • Liquid at room temperature – Waxes • Contains fatty acids and glycerol components – Glycerol – 3 carbon chain with an OH group bonded to each carbon – Fatty acid – chain of carbon atoms with a hydrogen or a carboxyl (-COOH) group bonded to each carbon • Dehydration (loss of water) combines 3 fatty acid molecules and 1 glycerol molecule to produce a fat or oil molecule • Primary function – reserve of stored energy • Examples: – Stored in fat cells of your body – Used on leaves as waxes to prevent water loss – Used to compose structural elements like honeycomb in a beehive (beeswax) • Saturated versus unsaturated fats: – Lipids can contain single or double bonds – Saturated fats • Solid at room temperature (fats or waxes) • Contain SINGLE bonds between carbon atoms – Unsaturated fats • Contain DOUBLE or TRIPLE bonds between carbon atoms • Liquids at room temperature (oils) • Polyunsaturated – more than one double bond – Doctors recommend more unsaturated and less saturated fats in your diet • Phospholipids – Plasma membrane component – Have hydrophobic and hydrophilic regions • Steroids – Building block for vitamin D and large hormones like testosterone and estrogen – Ex. Cholesterol Proteins • Composed of – Carbon – Hydrogen – Oxygen – Nitrogen • Amino Acids – Building block of a protein – Consist of a central carbon atom bonded to •1 •1 •1 •1 carboxyl group (COOH) amino group (NH2) hydrogen atom side chain (R) – different for every amino acid – 20 different amino acids • Simplest glycine (side chain is H) • Alanine CH3 side chain • Peptide Bond – Bond used to connect two amino acids together through dehydration synthesis – Forms between the amino group (-NH2) of one amino acid and the carboxyl group (-COOH) of another, resulting in the loss of one water molecule – Resulting molecule is called a dipeptide – Long chains of amino acids are called polypeptides – All proteins are made of one or more polypeptides bonded together • Protein structure (four levels) – Primary • Depends on what amino acids are used and how many – Secondary • Amino acid chain can fold into a helix or pleat – Tertiary • Considered to be globular or form long chains – Quaternary • Combination of long polypeptide chains to form a protein • The first protein structure identified was insulin, a hormone that controls blood glucose levels (1954) • Protein Function – Make up 15% of your body mass (10,000 different proteins in a single cell!) – Involved in almost every function of your body • Structural support • Cellular transport • Cell communication • Speeding up chemical reactions • Cell growth – Ex. Muscle, skin, hair, enzymes, hormones, antibodies Nucleic Acids • Composed of – – – – – Carbon Hydrogen Oxygen Nitrogen Phosphorus • Function – Store and transmit genetic information • Nucleotides – Basic structural unit – Contain a phosphate group (PO4), nitrogenous base and a 5-carbon sugar (ribose or deoxyribose) – Nitrogenous base is an organic base which contains nitrogen • Four types: –Adenine –Thymine –Cytosine –Guanine – Attached in a sequence along the length of the molecule (even up to 3 billion pairs!) • Two forms of nucleic acids in living things – DNA – Deoxyribonucleic acid • Hereditary material that is passed on from generation to generation through reproduction – RNA – Ribonucleic acid • Found within the cells nucleus – Directs and controls the development and activities of all the cells in an organism • Structure of DNA – Resembles the shape of a ladder • Two sides (sugarphosphate backbone) connected to each other by rungs (nitrogenous bases) – Double stranded – Sugar used is deoxyribose – DNA molecule is coiled into the form of a double helix • One human DNA molecule if stretched out straight could be 4 cm long! • Structure of RNA – Similar in chemical composition to DNA but consists of only one chain of bases attached to a sugar-phosphate backbone – Sugar used is ribose – The base thymine is replaced by uracil – RNA is involved in protein synthesis Main Ideas • Carbon compounds are the basic building blocks of living organisms • Biological macromolecules are formed by joining small carbon compounds into polymers • There are four types of biological macromolecules • Peptide bonds join amino acids in proteins • Chains of nucleotides form nucleic acids