* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Acid-Base Biochemistry
History of electrochemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Ocean acidification wikipedia , lookup
Atomic theory wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Water splitting wikipedia , lookup
Hydrogen bond wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Electrochemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Hydrogen atom wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Acid strength wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrolysis of water wikipedia , lookup
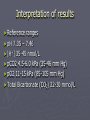
Acid-Base Biochemistry Dr. Catherine Street Consultant Clinical Biochemist Colchester Hospital university NHS Foundation Trust Acid-Base Biochemistry ► Definitions ► Methods ► Physiology ► Pathology Acid-Base Biochemistry Definitions What is an acid? What is a base? Acid-Base Biochemistry Definitions ► Definitions of an acid 1. Taste 2. Boyle 3. Arrhenius 4. Bronsted-Lowry 5. Lewis Acid-Base Biochemistry Definitions ► Taste Acere – tasting sour Lemon juice Vinegar Definition - Thousands of years old Acid-Base Biochemistry Definitions ► Robert Boyle 17th century ► Acids taste sour, are corrosive to metals, change litmus (a dye extracted from lichens) red, and become less acidic when mixed with bases (Alkali). ► Bases (Alkali) feel slippery, change litmus blue, and become less basic (alkaline) when mixed with acids. Acid-Base Biochemistry Definitions Arrhenius ► Arrhenius suggested that acids are compounds that contain hydrogen and can dissolve in water to release hydrogen ions into solution. For example, hydrochloric acid (HCl) dissolves in water as follows: HCl H2O + → H + Cl (g) (aq) (aq) Acid-Base Biochemistry Definitions ► Arrhenius defined bases as substances that dissolve in water to release hydroxide ions (OH-) into solution. For example, a typical base according to the Arrhenius definition is sodium hydroxide (NaOH): H2O NaOH (s) → Na+ (aq) + OH-(aq) Acid-Base Biochemistry Definitions ► The Arrhenius definition of acids and bases explains a number of things. Arrhenius's theory explains why all acids have similar properties to each other (and, conversely, why all bases are similar): because all acids release H+ into solution (and all bases release OH-). Acid-Base Biochemistry Definitions ► The Arrhenius definition also explains Boyle's observation that acids and bases counteract each other. This idea, that a base can make an acid weaker, and vice versa, is called neutralization. Acid-Base Biochemistry Definitions ► Neutralization: As you can see from the equations, acids release H+ into solution and bases release OH-. If we were to mix an acid and base together, the H+ ion would combine with the OH- ion to make the molecule H2O, or plain water: ► H+ (aq) + OH-(aq) → H2O Acid-Base Biochemistry Definitions ► The neutralization reaction of an acid with a base will always produce water and a salt, as shown below: ► Acid Base Water Salt ► HCl + NaOH → H2O + NaCl ► HBr + KOH → H2O + KBr Acid-Base Biochemistry Definitions ► Limitations of Arrhenius ► The Arrhenius definition does not explain why some substances, such as common baking soda (NaHCO3), can act like a base even though they do not contain hydroxide ions. Acid-Base Biochemistry Definitions Brǿnsted-Lowry 1923 An acid is any chemical species that donates a proton to another chemical species (proton donor) A base is any chemical species that accepts a proton from another chemical species (Proton acceptor) Acid-Base Biochemistry Definitions ► The Brønsted-Lowry definition of acids is very similar to the Arrhenius definition, any substance that can donate a hydrogen ion is an acid (under the Brønsted definition, acids are often referred to as proton donors because an H+ ion, hydrogen minus its electron, is simply a proton). Acid-Base Biochemistry Definitions ► The Brønsted definition of bases is, however, quite different from the Arrhenius definition. Arrhenius base releases hydroxyl ions whereas the Brønsted base is defined as any substance that can accept a hydrogen ion. Acid-Base Biochemistry Definitions ► The Brønsted-Lowry definition includes the Arrhenius bases so ► NaOH and KOH, as we saw above, would still be considered bases because they can accept an H+ from an acid to form water. ► But it extends the concept of a base and introduces the concept of conjugate acid-base pairs Acid-Base Biochemistry Definitions The removal of a proton (hydrogen ion) from an acid produces its conjugate base, which is the acid with a hydrogen ion removed, and the reception of a proton by a base produces its conjugate acid, which is the base with a hydrogen ion added Acid-Base Biochemistry Definitions ► The Brønsted-Lowry definition also explains why substances that do not contain OHions can act like bases. ► Baking soda (NaHCO3), for example, acts like a base by accepting a hydrogen ion from an acid as illustrated below: ► Acid Base Salt ► HCl + NaHCO3 → H2CO3 + NaCl Acid-Base Biochemistry Definitions ► Lewis definition 1923 ► A substance that can accept an electron pair from a base; thus, AlCl3, BF3, and SO3 are acids. ► The Lewis theory defines an acid as a species that can accept an electron pair from another atom, and a base as a species that can donate an electron pair to complete the valence shell of another atom Acid-Base Biochemistry Definitions pH Under the Brønsted-Lowry definition, both acids and bases are related to the concentration of hydrogen ions present. Acids increase the concentration of hydrogen ions, while bases decrease the concentration of hydrogen ions (by accepting them). The acidity or basicity of something therefore can be measured by its hydrogen ion concentration. Acid-Base Biochemistry Definitions ► In 1909, the Danish biochemist Sören Sörensen invented the pH scale for measuring acidity. The pH scale is described by the formula: ► pH = -log [H+] ► Note: concentration is commonly abbreviated by using square brackets, thus [H+] = hydrogen ion concentration. When measuring pH, [H+] is in units of moles of H+ per litre of solution. Acid-Base Biochemistry Methods pH electrode Acid-Base Biochemistry Methods ► pH electrode Acid-Base Biochemistry Methods How the pH Electrode works ► As the pH Glass comes into contact with an aqueous substance to measure, a gel layer forms on the membrane. This also happens on the inside of the glass layer. . Acid-Base Biochemistry Methods How the pH Electrode works ►The pH value of the aqueous solution will either force Hydrogen Ions out of the gel layer or into this layer. The Internal buffer in the glass electrode has a constant pH value and this keeps the potential at the inner surface of the membrane constant. Acid-Base Biochemistry Methods How the pH Electrode works ►The membrane potential is therefore the difference between the inner and outer charge. If you then factor in the stable potential of reference electrode, you have a voltage proportional to the pH value of the solution being measured, this being approximately 58mV/pH unit @ 20ºC Acid-Base Biochemistry Methods Other methods you need to know and understand ► Carbon dioxide electrode ► Oxygen electrode ► Laboratory measurement of bicarbonate ► Ion selective electrodes for K+ Na+ Cl- Acid-Base Biochemistry Physiology ► What is Physiological pH range? Acid-Base Biochemistry Physiology ► Extracellular fluid pH 7.35 – 7.46 (35-45 nmol/L) Does this apply to whole body ?any different pH ranges elsewhere Acid-Base Biochemistry Physiology More extreme/variable pH range Digestive tract Gastric Juice 1.0-3.0 Pancreatic Juice 8.0-8.3 Intercellular organelles Lysosomal pH 4-5 Digestive and lysosomal enzymes function optimally at these pH ranges Acid-Base Biochemistry Physiology Traditionally use pH to measure acidity Problem 1. direction of pH change is opposite to increase/decrease of Hydrogen ion concentration 2. Use of log scale ‘masks’ the extent of the change -change of 0.3 in pH represents doubling/halving of hydrogen ion concentration Acid-Base Biochemistry Physiology ► More recently – use Hydrogen ion concentration [H+] ► Traditionalists and older equipment use pH ► For large pH changes may not register change in units eg nmole/L to moles/L ► Most practical - give both Acid-Base Biochemistry Physiology ► WHAT BODY? THE SOURCES OF ACID IN THE Acid-Base Biochemistry Physiology ► Sources of acid Metabolism of food Metabolism of drugs Inborn errors of metabolism Acid-Base Biochemistry Physiology ► Acid production from metabolism of food Sulphuric acid from metabolism of sulphurcontaining amino acids of proteins Lactic acid from sugars Ketoacids from fats Acid-Base Biochemistry Physiology ► Acid production from metabolism of drugs Direct metabolism of drug to more acidic compound eg salicylates urates etc Induction of enzymes which metabolise other compounds (endogenous or exogenous) to acids Acid-Base Biochemistry Physiology ► Inborn errors of metabolism Organic acid disorders Lactic acidosis Acid-Base Biochemistry Physiology Greatest potential source of acid Carbon dioxide (1) CO2 + H2O <=> H2CO3 (2) H2CO3 <=> H+ + HCO3- Potentially 15,000 mmol/24 hours Acid-Base Biochemistry Physiology ► Hydrogen ion homeostasis ► 1. buffering ► 2. excretion Acid-Base Biochemistry Physiology Buffering of hydrogen ions In health as hydrogen ions are produced they are buffered – limiting the rise in [H+] Acid-Base Biochemistry Physiology Buffer solutions consist of a weak acid and its conjugate base As hydrogen ions are added some will combine with the conjugate base and convert it to undissociated acid Acid-Base Biochemistry Physiology Bicarbonate – carbonic acid buffer system H+ + HCO3- <=> H2CO3 ► Addition of H+ drives reaction to the right Conversely ► Fall in H+ drives reaction to the left as carbonic acid dissociates producing more H+ Acid-Base Biochemistry Physiology ► Buffering systems in blood Bicarbonate ions-most important Proteins including intracellular proteins Haemoglobin Acid-Base Biochemistry Physiology ► Buffer solutions operate most efficiently at [H+] that result in approximately equal concentration of undissociated acid and conjugate base ► But at normal extracellular fluid pH [H2CO3] 1.2 mmol whereas [HCO3-] is twenty times greater Acid-Base Biochemistry Physiology ► The bicarbonate system is enhanced by the fact that carbonic acid can be formed from CO2 or disposed of by conversion to CO2 CO2 + H2O <=> H2CO3 Acid-Base Biochemistry Physiology ► For every hydrogen ion buffered by bicarbonate – a bicarbonate ion is consumed. ► To maintain the capacity of the buffer system, the bicarbonate must be regenerated ► However, when bicarbonate is formed from carbonic acid (CO2 and H2O) equimolar amounts of [H+] are formed Acid-Base Biochemistry Physiology ► Bicarbonate formation can only continue if these hydrogen ions are removed ► This process occurs in the cells of the renal tubules where hydrogen ions are secreted into the urine and where bicarbonate is generated and retained in the body Acid-Base Biochemistry Physiology ►2 different processes ► Bicarbonate regeneration (incorrectly reabsorption) ► Hydrogen ion excretion Acid-Base Biochemistry Physiology Importance of Renal Bicarbonate Regeneration ► Bicarbonate is freely filtered through the glomerulus so plasma and glomerular filtrate have the same bicarbonate concentration ► At normal GFR approx 4300 mmol of bicarbonate would be filtered in 24 hr ► Without re-generation of bicarbonate the buffering capacity of the body would be depleted causing acidotic state ► In health virtually all the filtered bicarbonate is recovered Acid-Base Biochemistry Physiology ► Renal Bicarbonate Regeneration involves the enzyme carbonate dehydratase (carbonic anhydrase) ► Luminal side of the renal tubular cells impermeable to bicarbonate ions ► Carbonate dehydratase catalyses the formation of CO2 and H2O from carbonic acid (H2CO3) in the renal tubular lumen ► CO2 diffuses across the luminal membrane into the tubular cells Acid-Base Biochemistry Physiology ► ► ► ► ► within the renal tubular cells carbonate dehydratase catalyses the formation of carbonic acid (H2CO3) from CO2 and H2O Carbonic acid then dissociates into H+ and HCO3The bicarbonate ions pass into the extracellular fluid and the hydrogen ions are secreted back into the lumen in exchange for sodium ions which pass into the extracellular fluid Exchange of sodium and hydrogen ions an active process involving Na+/K+/H+ ATP pump K+ important in electrolyte disturbances of acid-base Acid-Base Biochemistry Physiology ► ► ► ► ► ► Regeneration of bicarbonate does not involve net excretion of hydrogen ions Hydrogen ion excretion requires the same reactions occurring in the renal tubular cells but also requires a suitable buffer in urine Principal buffer system in urine is phosphate 80% of phosphate in glomerular filtrate is in the form of the divalent anion HPO42This combines with hydrogen ions HPO42- + H+ ↔ H2PO4- Acid-Base Biochemistry Physiology ► Hydrogen ion excretion capacity ► The minimum urine pH that Can be generated is 4.6 ( 25µmol/L) ► Normal urine output is 1.5L ► Without the phosphate buffer system the free excretion of Hydrogen ions is less than 1/1000 of the acid produced by normal metabolism Acid-Base Biochemistry Physiology ► The phosphate buffer system increases hydrogen ion excretion capacity to 30-40 mmol/24 hours ► In times of chronic overproduction of acid another urine buffer system ► Ammonia Acid-Base Biochemistry Physiology ► Ammonia produced by deamination of glutamine in renal tubular cells ► Catalysed by glutaminase which is induced by chronic acidosis ► Allows increased ammonia production and hence increased hydrogen ion excretion via ammonium ions ► NH3 + H+ ↔ NH4+ Acid-Base Biochemistry Physiology ► ► ► ► At normal intracellular pH most ammonia is present as ammonium ions which can’t diffuse out of the cell Diffusion of ammonia out of the cell disturbs the equilibrium between ammonia and ammonium ions causing more ammonia to be formed Hydrogen ions formed at the same time! These are used up by the deamination of glutamine to glutamate during gluconeogenesis Acid-Base Biochemistry Physiology ► Carbon dioxide transport ► Carbon dioxide produced by aerobic respiration diffuses out of cells and into the ECF ► A small amount combines with water to form carbonic acid decreasing the pH of ECF ► In red blood cells metabolism is anaerobic and very little CO2 is produced hence it diffuses into red cells down a concentration gradient to form carbonic acid (carbonate dehydratase) buffered by haemoglobin . Acid-Base Biochemistry Physiology ► Haemoglobin has greatest buffering capacity when it is dexoygenated hence the buffering capacity increases as oxygen is lost to the tissues ► Net effect is that carbon dioxide is converted to bicarbonate in red cells ► Bicarbonate diffuses out of red cells down concentration gradient and chloride ions diffuse in to maintain electrochemical neutrality (chloride shift) •Acid-Base Biochemistry Physiology ► In the lungs this process is reversed ► Haemoglobin is oxygenated reducing its buffering capacity and generating hydrogen ions ► These combine with bicarbonate to form CO2 which diffuses into the alveoli ► Bicarbonate diffuses into the cells from the plasma Acid-Base Biochemistry Physiology ► Most of the carbon dioxide in the blood is present as bicarbonate ► Carbon dioxide, carbonic acid and carbamino compounds less than 1/10 th of the total ► Bicarbonate /total CO2 used interchangeably though not strictly the same ► Most analytical methods actually measure total CO2 as bicarbonate difficult to measure Acid-Base Biochemistry Physiology The hydrogen ion concentration of plasma is directly proportional to the PCO2 and inversely proportional to bicarbonate [H+] = k pCO2/[HCO3-] [H+] in nmoles/L, [HCO3-] in mmoles/L pCO2 in kPa k = 180 pCO2 in mm Hg k= 24 Acid-Base Biochemistry Physiology ► Derived bicarbonate ► Possible to use the equation to calculate the bicarbonate concentration from the pCO2 and pH (blood gas analysers) ► ?how valid in non-ideal solutions ► Auto analysers – measured bicarbonate Acid-Base Biochemistry Physiology ► The relationship between [H+], pCO2 and bicarbonate fundamental to understanding pathophysiology of hydrogen ion homeostasis Acid-Base Biochemistry Pathology ►4 Components to acid-base disorders Generation Buffering Compensation Correction Occurring concurrently Acid-Base Biochemistry Pathology ► Classification of acid-base disorders ► Acidosis ► [H+] above normal, pH below normal ► Alkalosis ► [H+] below normal, pH above normal Acid-Base Biochemistry Pathology ► Further classified as Respiratory Non-respiratory (metabolic) Mixed – difficult to distinguish between primary mixed condition and compensated disorder Acid-Base Biochemistry Pathology ► Respiratory disorders involve a change in pCO2 ► Metabolic disorders involve change in production or excretion of hydrogen ions or both Acid-Base Biochemistry Pathology ► Non-respiratory acidosis ► Increased production/reduced excretion of acid ► ?causes Acid-Base Biochemistry Pathology ► Non-respiratory acidosis ► Overproduction of acid Keto acidosis (diabetes, starvation, alcohol) Lactic acidosis (inherited metabolic defect or drugs) Inherited organic acidoses Poisoning (salicylate, ethylene glycol, alcohol) Excessive parenteral amino acids Acid-Base Biochemistry Pathology ► Non-respiratory acidosis ► Reduced excretion of acid Generalised renal failure Renal tubular acidoses Carbonate dehydratase inhibitors Acid-Base Biochemistry Pathology ► Non-respiratory acidosis ► Loss of Bicarbonate Diarrhoea Pancreatic, intestinal, biliary fistula or drainage Acid-Base Biochemistry Pathology ► Compensation of non-respiratory acidosis Excess hydrogen ions are buffered by bicarbonate forming carbonic acid which dissociates to carbon dioxide to be lost in expired air The buffering limits the rise in [H+] at the expense of reduction in bicarbonate Acid-Base Biochemistry Pathology ► Compensation of non-respiratory acidosis ► Hyperventilation increases removal of CO2 lowering pCO2 ► PCO2 / [HCO3-] ratio falls reducing [H+] ► Hyperventilation is the direct result of increased [H+] stimulating the respiratory centre (Kussmaul respiration) Respiratory compensation of non-respiratory acidosis Acid-Base Biochemistry Pathology ► Compensation of non-respiratory acidosis ► Limitations ► Respiratory compensation cannot completely normalise the [H+] because the hyperventilation is stimulated by the increase in [H+] and as this falls the drive on the respiratory centre is reduced ► Increased work of respiratory muscles during hyperventilation produces CO2 limiting the degree to which PCO2 can be lowered Acid-Base Biochemistry Pathology ► The degree of compensation may be limited further if respiratory function is compromised ► If it is not possible to correct the cause of the acidosis may get a new steady state of chronic acidosis [H+] [HCO3-] and ↓PCO2 Acid-Base Biochemistry Pathology ► In the absence of acidosis - hyperventilation would normally generate a respiratory alkalosis ► Compensatory mechanisms usually involve generation of a second opposing disturbance ► In non-respiratory acidosis the hyperventilation limits the severity of the acidosis but is not great enough to cause alkalosis in the patient Acid-Base Biochemistry Pathology ► Non-respiratory compensation of nonrespiratory acidosis ► If renal function is normal excess [H+] can be excreted by the kidneys ► But renal function is often impaired even if not the primary cause of the acidosis Acid-Base Biochemistry Pathology ► Correction of acidosis ► Complete correction requires reversal or removal of the underlying cause ► Ethylene glycol poisoning – slow the rate of metabolism with ethanol ► Diabetes – rehydration and insulin Acid-Base Biochemistry Pathology ► Summary of non-respiratory acidosis ► [H+] ► PCO2 ► pH ► [HCO3-] Acid-Base Biochemistry Pathology ► Management of non-respiratory acidosis ► 1. Removal of cause ► 2. Administration of Bicarbonate – only in severe cases pH <7.0 and where 1 is not possible ► Must be given in small quantities with constant monitoring of pH Acid-Base Biochemistry Pathology ► Respiratory acidosis ► Primarily an increase in PCO2 ► Number of different causes Acid-Base Biochemistry Pathology ► Retention of CO2 ► Production of carbonic acid ► For every hydrogen ion produced a bicarbonate ion is generated ► Most of the [H+] is buffered by intracellular buffers (haemoglobin) ► Development of renal compensation if renal function is normal Acid-Base Biochemistry Pathology ► Acute respiratory acidosis For every KPa increase in PCO2 increase in bicarbonate < 1 mmole Increase in [H+] 5.5 nmol/L ► Chronic For every KPa increase in PCO2 increase in bicarbonate 2-3 mmole Increase in [H+] 2.5 nmol/L Acid-Base Biochemistry Pathology ► Compensation of respiratory acidosis ► Increased renal excretion of hydrogen ions Acid-Base Biochemistry Pathology ► Management of respiratory acidosis ► With reduced ventilation it is usually the hypoxaemia that is life threatening 4 mins if ventilation ceases ► Improve alveolar ventilation bronchodilators and antibiotics ► Artificial ventilation close monitoring required to avoid over correction esp in chronic acidosis Acid-Base Biochemistry Pathology Summary of respiratory acidosis Acute Chronic pH [H+] PCO2 Slight or low normal Slight or high normal [HCO3-] Slight Acid-Base Biochemistry Pathology ► Non respiratory alkalosis ► Loss of un-buffered hydrogen ions Gastrointestinal - vomiting with pyloric stenosis - diarrhoea - nasogastric aspiration Acid-Base Biochemistry Pathology Causes of non respiratory alkalosis Renal Mineralo-corticoid excess Conn’s syndrome Cushings syndrome Drugs with mineralocorticoid activity Diuretic therapy (not K+ sparing) Acid-Base Biochemistry Pathology Causes of non respiratory alkalosis Administration of alkali Over-treatment of acidosis Chronic alkali ingestion (antacids) Acid-Base Biochemistry Pathology ► Non respiratory alkalosis ► Characterised by primary increase in ECF bicarbonate ► Consequent reduction in [H+] ► Normally increase in bicarbonate causes reduction in renal bicarbonate regeneration and increased urinary excretion of bicarbonate Acid-Base Biochemistry Pathology ► non respiratory alkalosis ► Maintenance requires inappropriate renal bicarbonate reabsorption/regeneration - decrease in ECF volume (hypovolaemia) - mineralocorticoid excess - potassium depletion Acid-Base Biochemistry Pathology ► non respiratory alkalosis ► Hypovolaemia Increased stimulus to sodium reabsorption Dependant on adequate anions If chloride deficient (GI losses) electrochemical neutrality during Na+ absorption maintained by increased bicarbonate absorption and by H+ and K+ excretion Acid-Base Biochemistry Pathology ► non respiratory alkalosis ► Mineralocorticoid excess Alkalosis perpetuated by increased hydrogen ion excretion secondary to increased sodium reabsorption Potassium depletion Potassium and hydrogen ion excretion compete for exchange with sodium so depletion of potassium causes increased H+ excretion Acid-Base Biochemistry Pathology non respiratory alkalosis ► Compensation ► Low H+ inhibits the respiratory centre causing hypoventilation and increase in PCO2 ► Self- limiting as increase in PCO2 increases drive on respiratory centre ► In chronic state development of reduced sensitivity to PCO2 – more significant compensation BUT ► Hypoventilation causing hypoxaemia will provide stimulation of RC and prevent further compensation ► Acid-Base Biochemistry Pathology ► non respiratory alkalosis ► Management ► Dependent on severity and cause ► - severe hypovolaemia /hypochloraemia correct with saline infusion ► - potassium supplements/removal of diuretics Acid-Base Biochemistry Pathology ► Summary of non respiratory alkalosis ► pH ► PCO2 ► [HCO3-] ► [H+] Acid-Base Biochemistry Pathology ► Respiratory alkalosis ► Causes ► Hypoxia High altitude Severe anaemia Pulmonary disease Acid-Base Biochemistry Pathology ► Respiratory alkalosis ► Causes ► Increased respiratory drive Stimulants eg salicylates Cerebral – trauma, infection, tumours Hepatic failure Acid-Base Biochemistry Pathology ► Respiratory alkalosis ► Causes Pulmonary disease - Pulmonary oedema - Pulmonary embolism Mechanical over-ventilation Acid-Base Biochemistry Pathology ► Respiratory alkalosis ► Characterised by reduction in PCO2 ► Reduces the PCO2/ [HCO3-] ratio For every KPa decrease in PCO2 decrease in [H+] 5.5 nmol/L Small decrease in bicarbonate Acid-Base Biochemistry Pathology ► Respiratory alkalosis ► Compensation -reduction in renal hydrogen ion excretion Develops slowly maximal in 36-72 hours Acid-Base Biochemistry Pathology ► Respiratory alkalosis management ► Mainly removal of underlying cause ► Increasing inspired PCO2 by rebreathing of expired air for temporary measure - Prolonged – risk of hypoxia Acid-Base Biochemistry Pathology ► Summary of respiratory alkalosis ► Acute Chronic ► pH Slight or low normal ► [H+] Slight or high normal ► PCO2 ► [HCO3-] Slight Acid-Base Biochemistry Pathology ► Mixed acid base disorders respiratory alkalosis with metabolic acidosis e.g. salicylate poisoning causes respiratory alkalosis by directly stimulating the hypothalamic respiratory centre causing over-breathing and increased excretion of CO2 Salicylate metabolised to acids Interpretation of results ► Reference ranges ► pH 7.35 – 7.46 ► [H+] 35-45 nmol/L ► pCO2 4.5-6.0 kPa (35-46 mm Hg) ► pO2 11-15 kPa (85-105 mm Hg) ► Total Bicarbonate (CO2) 22-30 mmol/L Further information A further algorithm for interpretation of acid-base data and a number of clinical cases were provided as hard-copy. These can be found in Marshall (see recommended reading) Acid-Base Biochemistry Methods Acid-Base Biochemistry Methods ► ► ► ► ► The polarographic (Clark) oxygen electrode measures the oxygen partial pressure in a blood or gas sample. A platinum cathode and a silver/silver chloride anode are placed in a sodium chloride electrolyte solution, and a voltage of 700 mv is applied (Figure 1). The following reactions occur. At the cathode: O2 + 2H2O + 4e– = 4OH–. In the electrolyte: NaCl + OH– = NaOH + Cl–. At the anode: Ag + Cl– = AgCl + e–. Electrons are taken up at the cathode and the current generated is proportional to oxygen tension. A membrane separates the electrode from blood, preventing deposition of protein but allowing the oxygen tension in the blood to equilibrate with the electrolyte solution. The electrode is kept at a constant temperature of 37°C and regular checks of the membrane are required to ensure it is not perforated or coated in proteins. Sampling two gas mixtures of known oxygen tension allows calibration. Acid-Base Biochemistry Methods ► The Severinghaus or carbon dioxide electrode is a modified pH electrode in contact with sodium bicarbonate solution and separated from the blood specimen by a rubber or Teflon semipermeable membrane. Carbon dioxide, but not hydrogen ions, diffuses from the blood sample across the membrane into the sodium bicarbonate solution, producing hydrogen ions and a change in pH. ► Hydrogen ions are produced in proportion to the pCO2 and are measured by the pH-sensitive glass electrode. As with the pH electrode, the Severinghaus electrode must be maintained at 37°C, be calibrated with gases of known pCO2 and the integrity of the membrane is essential. Because diffusion of the CO2 into the electrolyte solution is required the response time is slow at 2–3 minutes. Acid-Base Biochemistry Methods ION SELECTIVE ELECTRODE Acid-Base Biochemistry RECOMMENDED READING ► Analytical/methods Tietz Textbook of Clinical Chemistry by Carl A. Burtis (Author), Edward R. Ashwood (Author) ► Clinical Clinical Biochemistry by William J. Marshall and Stephen Bangert