* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CHAPTER 2
Survey
Document related concepts
Orphan drug wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Compounding wikipedia , lookup
Pharmacognosy wikipedia , lookup
Plateau principle wikipedia , lookup
List of comic book drugs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Drug design wikipedia , lookup
Drug discovery wikipedia , lookup
Transcript
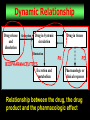
CHAPTER 2 DEFINITIONS RELATED TO PHARMACOKINETICS 1 PHARMACY The Pharmacist has to learn: What physiological factors affect drug release To calculate fraction of the dose of drug absorbed To learn PK to calculate the rate of absorption Patients counseling and physicians advising about drug products and their use (clinical pharmacist) 2 PHAMACOKINETICS (PK) The WHO defines PK as: “The study of the absorption, distribution, metabolism, and excretion of drugs”. This definition lacks: “The concept that you have to apply mathematics and modeling to data collected in studies of absorption, distribution and elimination of drugs before PK is involved”. 3 PURPOSE of PK In PK the data are processed using a mathematical representation of a part or the whole of an organism To reduce data to meaningful numbers of parameters To use the reduced data to make predictions of results of future experiments 4 How We Study PK of Drugs? The study involves and Experimental and a Theoretical approach. Experimental part consists of: Biological sampling techniques, analytical methods, data collection and manipulation. Theoretical part consists of: Development of PK models that predict drug disposition after administration. 5 CLINICAL PK “The application of PK to the safe and effective therapeutic management of the individual patient”. Its functions include: Design of drug dosage regimens Readjustment of dosage regimens when necessary Investigate the reason behind an unusual response Educational activities directed to physicians, pharmacists, nurses, and patients. 6 PHARMACODYNAMICS (PD) Refers to the relationship between the drug concentration at the site of action (receptor) and pharmacologic or toxic response. PK-PD Models are constructed to relate the plasma drug level to the concentration of the drug in the site of action and establish the intensity and time course of the drug. 7 BIOPHARMACEUTICS Considers the interrelationship of the physicochemical properties of the drug, the dosage form, and the route of administration on the rate and extent of systemic drug absorption Biopharmaceutics Methods Must be able to assess the impact of the physico-chemical properties of the drug, drug stability, and large scale production of the drug and drug product on the biological performance of the drug 8 Dynamic Relationship Drug release and dissolution Absorption Drug in Systemic circulation Elimination BIOPHARMACEUTICS Excretion and metabolism Drug in tissues PK PD Pharmacologic or clinical response Relationship between the drug, the drug product and the pharmacologic effect 9 BIOAVAILABILITY (F) “is defined as the fraction of the administered dose reaching the systemic circulation of the patient”. Factors Affecting BA: Inherent dissolution and absorption characteristics of the administered chemical form (salt, ester), the dosage form (tablet, solution,..), the route of administration, the stability of the drug in the GI tract, and the extent of drug metabolism. 10 First-Pass Effect Refers to the loss of drug as it passes for the first time through organs of elimination such as the GI membrane and liver during the drug absorption process. Portal Circulation Gut Wall Gut Lumen L i v e r 11 Intravascular administration • All rout of administration where the drug is directly introduced in to blood stream. Intravenous, intra arterial. And intra cardial. • Extra vascular, All rout of administration, Im, Sc, oral, rectal, topical, trans dermal ,etc. 12 Plasma level time curve • Is generated by obtaining the drug concentration in plasma samples taken at various time intervals after a drug product is administered. • The concentration of drug in each plasma sample is plotted on rectangular coordinate graph paper against the corresponding time at which the plasma sample was removed 13 Blood flow rate • The speed of blood perfusion in an organ, usually expressed in ml/100gm organ weight. Blood flow rates may differ several-folds between rest and exercise. 14 Definitions you should know • Pharmacokinetics, intravascular,extravascular • Absorption (process by which a drug proceeds from the site of administration to the site of measurement within the body. • Disposition(all the processes that occur subsequent to the absorption of a drug • Distribution ; reversible transfer of a drug to and from the site of measurement. • Metabolism; irreversible conversion to another chemical species. • Excretion; irreversible loss of the chemically unchanged drug 15 • Accumulation; the increase of drug concentration in blood and tissue upon multiple dosing until steady state is reached • Steady state; the level of drug accumulation in blood and tissue upon multiple dosing when input and output are at equilibrium . • Biophase;the actual site of action of drug in the body. • A receptor; a site in the biophase to which drug molecules can be bound • A compartment in pharmacokinetics; an entity which can be described by adefinite volume and concentration of drug contained in that volume. In pharmacokinetics , experimental data are explained by fitting them to compartmental models. • Central compartment; the sum of all body regions( organs and tissue) in which the drug concentration is in instantaneous equilibrium with that in blood or plasma. The blood or plasma is always part of the central compartment • Peripheral compartment; the sum of all body regions to which a drug is eventually distributes but is not in instantaneous equilibrium. 16 • Feathering ; refers to a graphical method for separation of exponents such as separating the absorption rate constant from the elimination rate constant. ( residual method) • Biliary recycling; the phenomenon that drugs emptied via bile in to the small intestine can be reabsorbed from the intestinal lumen in to systemic circulation. • Apparent partition coefficient; the ratio of the concentration at equilibrium between a lipoid phase (n, octane) and an aqueous phase ( buffer ph 7.4). • Area under the curve; the integral of drug level over time from zero to infinity, and Isa measure of the quantity of drug absorbed in the body. • Clearance rate; the volume of blood in ml which is completely cleared of the drug per unit time (minute) by urinary excretion or metabolism. • Renal clearance; the hypothetical plasma volume (volume of plasma volume (volume of of unmetabolized drug which is cleared in one minute via the kidney. • Hepatic clearance; the hypothetical plasma volume (volume of distribution) in ml of the metabolized drug which is cleared in one minute via the liver 17 Significance of measuring plasma drug concentration • Checking the plasma drug level is a responsive method of monitoring the course of therapy • Monitoring the concentration of drugs in blood or plasma ascertain that the calculated dose actually deliver drug required for therapeutic effect. plasma • Is needed to distinguish thevolume patient(volume who isof receiving too much drug from the patient who is supersensitive to the drug. • Allows for the adjustment of the drug dosage in order to individualize and optimize therapeutic drug regimens. • Provide a guide to the progress of the diseased state and enable the investigator to modify the drug dosage accordingly(in clinical pharmacokinetics). 18