* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
5-HT2C receptor agonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
NMDA receptor wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Transcript
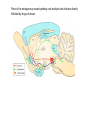
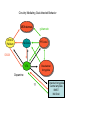
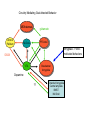
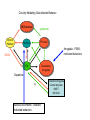
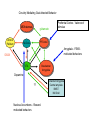
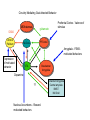
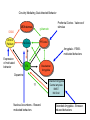
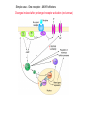
Topics for this lecture Drugs - All drugs of abuse induce dopamine release (?) The anatomy of the reward pathway defines the road to drug abuse (?) Cellular adaptations to drugs of abuse - not so impressive - are they important? Systems adaptations to drugs of abuse - more impressive - way more complicated! Conclusion - Many drugs of abuse act on receptors that endogenous 'transmitters' also activate Drug Endogenous ligands Action on Nicotine THC Opioids Acetylcholine anandamide, 2AG Enk,ß-endorphin, Dyn Ligand gated channels Cannabinoid receptors (GPCRs) Opioid GPCRs (µ,∂,k) Cocaine Amphetamine MDMA (ecstasy) 5-HT, DA, NA transporters DA,NA transporters, VMAT (therefore multiple GPCRs) 5-HT transporters, VMAT Three classes of drugs act on separate effectors that all increase the release of dopamine Class 1: GPCRs - opioids, cannabinoids, GHB Class 2: Ionotropic receptors/ion channels - nicotine, alcohol Class 3: Transporters - cocaine, methamphetamine, ecstasy Class 1: GPCRs - opioids 1. g-i coupled receptors 2. Best known acute actions are ALL inhibitory a. Increase Potassium Conductance b. Decrease Calcium Conductance c. Decrease Transmitter Release By inhibiting neurons that release GABA - opioids can result in excitation (dis-inhibition) - first described in hippocampus (1979) and is though to happen in many areas including dopamine cells of the VTA. 3. 4. Opioids increase dopamine release by dis-inhibiting dopamine neurons! As with all G-Protein Coupled receptors - There is much more going on with opioid receptor activation. We will discuss some of the downstream effectors. Opioid inhibition of GABA IPSCs in different dopamine cells in the VTA (inhibition of IPSCs results in less inhibition of dopamine cells) Opioid inhibition of GABA-B IPSCs on different dopamine cells Class 2: Ionotropic receptors/ion channels - nicotine 1. 2. 3. 4. 5. Nicotinic acetylcholine receptors - multiple subunits, pentameric Cation non-selective ion channel Simple to see how the activation of these receptors will result in an increase in excitability of dopamine cells. These receptors are also found on presynaptic terminals, including the terminals of dopamine cells. Activation of nicotinic receptors increases dopamine release (transmitters at other terminals too). One confounding problem in the dopamine system is the fact that GABA neurons (inter-neurons) also have nicotinic receptors. Thus under some conditions nicotine can decrease the activity of dopamine cells by causing an increased release of GABA. Figure 1. Wooltorton, J. R. A. et al. J. Neurosci. 2003;23:3176-3185 Figure 6. Wooltorton, J. R. A. et al. J. Neurosci. 2003;23:3176-3185 Copyright ©2003 Society for Neuroscience Class 3: Transporters - cocaine, methamphetamine 1. 2. 3. 4. 5. Cocaine blocks all monoamine transporters - dopamine, noradrenaline, 5-HT Amphetamine and methamphetamine are substrates for the dopamine and noradrenaline transporters, different from cocaine. Amphetamine and methamphetamine are substrates for the vesicular monoamine transporter - and thus deplete vesicular stores of monoamines. These simple and straight forward mechanisms lead to VERY complicated changes in downstream signaling. Think about all the subtypes of monoamine receptors and how many things would be affected by an increase in the extracellular levels of all monoamines. Dopamine release in projection areas is dramatically increased. One confounding problem is that the extracellular level of dopamine is also increased in the dopamine cell body region. That results in the activation of inhibitory dopamine receptors (D2) that decrease the activity of dopamine cells. How does this line up with an increase in dopamine release in projection areas?? Cocaine increases the size and the time course of the cloud of dopamine. Control - localized diffusion Cocaine - extended diffusion Dopamine transporter D2 receptor Dopamine IPSC on a dopamine cell in the VTA The dopamine transporter is the primary mechanism that terminates dopamine signaling. Parts of the endogenous reward pathway and multiple sites that are directly Affected by drugs of abuse. Circuitry Mediating Goal-directed Behavior MDthalamus Ventral Pallidum glutamate PFCortex NA Core GABA VTA Basolateral Amygdala Dopamine ?? Extended Amygdala Central amydala BNST NA Shell Circuitry Mediating Goal-directed Behavior MDthalamus Ventral Pallidum glutamate PFCortex NA Core Amygdala - FEARmotivated behaviors GABA VTA Basolateral Amygdala Dopamine ?? Extended Amygdala Central amydala BNST NA Shell Circuitry Mediating Goal-directed Behavior MDthalamus Ventral Pallidum glutamate PFCortex NA Core Amygdala - FEARmotivated behaviors GABA VTA Basolateral Amygdala Dopamine ?? Nucleus Accumbens - Rewardmotivated behaviors Extended Amygdala Central amydala BNST NA Shell Circuitry Mediating Goal-directed Behavior MDthalamus Ventral Pallidum glutamate Prefrontal Cortex - Valence of stimulus PFCortex NA Core Amygdala - FEARmotivated behaviors GABA VTA Basolateral Amygdala Dopamine ?? Nucleus Accumbens - Rewardmotivated behaviors Extended Amygdala Central amydala BNST NA Shell Circuitry Mediating Goal-directed Behavior MDthalamus glutamate GABA Ventral Pallidum Prefrontal Cortex - Valence of stimulus PFCortex NA Core Amygdala - FEARmotivated behaviors Expression of motivated behavior VTA Basolateral Amygdala Dopamine ?? Nucleus Accumbens - Rewardmotivated behaviors Extended Amygdala Central amydala BNST NA Shell Circuitry Mediating Goal-directed Behavior MDthalamus glutamate GABA Ventral Pallidum Prefrontal Cortex - Valence of stimulus PFCortex NA Core Amygdala - FEARmotivated behaviors Expression of motivated behavior VTA Basolateral Amygdala Dopamine ?? Nucleus Accumbens - Rewardmotivated behaviors Extended Amygdala Central amydala BNST NA Shell Extended Amygdala - Stressorinduced behaviors Circuitry Mediating Goal-directed Behavior MDthalamus Ventral Pallidum glutamate PFCortex NA Core GABA VTA Dopamine from the VTA goes to each part of the circuit. 1. Alerts to novel stimulus 2. Alerts to learned associations Basolateral Amygdala ?? Extended Amygdala Central amydala BNST NA Shell Drug Seeking = Disruption of the Motive Circuit Components critical for craving and drug seeking Final common pathway Ventral Pallidum PFCortex NA Core cue VTA stress Basolateral Amygdala Extended Amygdala Central amydala BNST NA Shell How were the disruptions studied 1. Inactivation of specific nuclei 2. Testing for reinstatement of drug seeking 3. Examining cerebral blood flow/O2 utilization Drug Seeking = Disruption of the Motive Circuit Components critical for craving and drug seeking Final common pathway Ventral Pallidum PFCortex NA Core cue VTA stress Basolateral Amygdala Extended Amygdala Central amydala BNST NA Shell Three general principles 1. Final common pathway - AMPA receptor in N.Accumbens 2. Modality-dependent stimuli - Cue/Stress/Drug induced 3. Mesocorticolimbic dopamine release Drug Seeking = Disruption of the Motive Circuit Components critical for craving and drug seeking Final common pathway Ventral Pallidum PFCortex NA Core cue VTA stress Basolateral Amygdala Extended Amygdala Central amydala BNST NA Shell Stages of addiction 1. Acute Drug Effects - this is what we know the MOST about 2. Transition to Addiction - transcriptional regulators - protein expression 3. End-Stage Addiction - enduring changes ?? Acute actions of opioids on neurons Increase Potassium Conductance Decrease Calcium Conductance Decrease Transmitter Release Long-term actions of opioids on neurons Acute desensitization Receptor down regulation How do the acute and long-term actions of opioids result in addiction? Acute - receptor desensitization/internalization Chronic - receptor down regulation If the decline was ONLY the result of desensitization The after-reaction is the result of counter-adaptation(s) someplace beyond the receptor. When the agonist is removed the counter-adaptation takes time to re-adjust. During the re-adjustment time the system is not at baseline (withdrawal). There are MANY stages of withdrawal from drugs - some last forever Simple case - One receptor - MANY effectors Changes induced after prolonged receptor activation (red arrows) Pre-synaptic inhibition by morphine is increased during withdrawal WHY?? Chronic morphine treatment Up-regulates an opioid sensitive adenylyl cyclase. The increased cAMP increases transmitter release. The increased transmitter release is sensitive to morphine During withdrawal morphine inhibits transmission by two mechanisms What are the links from the acute cellular effects of drugs and the development of addiction? With repeated administration of drugs - there is a rewiring of key synapses. Could this be mediated by the same processes that underlie learning and memory (Synaptic plasticity - LTP/LTD)?? Dopamine cells in the VTA The ratio of currents induced by AMPA and NMDA receptors can be used as an indication of a change in synaptic plasticity. The AMPA/NMDA is low in dopamine cells in control animals. After treatment of animals with drugs the ratio is increased. The results suggests that the drug treatment resulted in a LTP type change in transmission. The same result was obtained after treatment of animals with MANY drugs of abuse. What does this mean for the activity of dopamine cells? What are potential mechanisms that would account of this result? Animals will self-administer drugs Results obtained from animals that self-administer drugs can be compared with animals that receive the same drug given passively This experiment will distinguish pure pharmacology from the systems level desire for the drug. Bed Nucleus of the Stria Terminals is a site known to be involved in relapse to drug seeking Behavior. The AMPA/NMDA was increased ONLY in animals that self-administered either cocaine or a food reward. Drug abuse A lot is known about how individual drugs of abuse act. 1. Which receptors 2. What second messengers 3. How gene expression may be changed Some drugs of abuse are also used therapeutically (examples) There are only theories about why these are drugs of abuse. Theories by big names in the field - Wise, Koob 1. Some take drugs to feel good (activating endogenous reward) 2. Some take drugs to stop feeling bad (correct a neurochemical deficit) Most people can use drugs with little or no long-lasting problems The lives of some are dominated by drugs of abuse. Why are some people affected in others not? Is it the pharmacology of that drug in that person that is different? Is it the brain neurochemistry/neurobiology that is different?