* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Orphan drug wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Compounding wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
List of comic book drugs wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Drug discovery wikipedia , lookup
Plateau principle wikipedia , lookup
Transcript
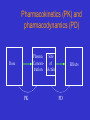
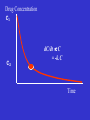
Clinical Pharmacokinetics Introduction How to use this powerpoint presentation • This supplements the other course material • You can view it on line or download it to your computer and view it without being connected to the internet. • Work through the presentation at the start of the course and note any issue which are not clear. • Read up on areas that you are not familiar with and revisit the presentation from time to time. • Try the powerpoint based exercises What is clinical pharmacokinetics ? • Study of the time course of a drug’s movement through the body. • Understanding of what the body does to (or with) the drug. • Application of Therapeutic Drug Monitoring (TDM) and individualisation of drug therapy. Outline • Review of Concepts – Clearance, K, Half-Life, Volume of Distribution • Therapeutic drug Monitoring • Pharmacokinetic Drug Interactions • Cases • Discussion/Questions Pharmacokinetics (PK) & pharmacodynamics (PD) • PK - What the body does to the drug? – Absorption; distribution, metabolism, excretion (ADME) • PD - What the drug does to the body? – Drug concentration at the site of action or in the plasma is related to a magnitude of effect Pharmacokinetics (PK) and pharmacodynamics (PD) Plasma Concentration Dose PK Site of Action Effects PD Pharmacokinetics vs Pharmacodynamics…concept • Fluoxetine increases plasma concentrations of amitriptyline. This is a pharmacokinetic drug interaction. • Fluoxetine inhibits the metabolism of amitriptyline and increases the plasma concentration of amitriptytline. Pharmacokinetics vs Pharmacodynamics…concept • If fluoxetine is given with tramadol serotonin syndrom can result. This is a pharmacodynamic drug interaction. • Fluoxetine and tramadol both increase availability of serotonin leading to the possibility of “serotonin overload” This happens without a change in the concentration of either drug. Basic Parameters • In the next few slides the basic concepts and paramaters will be described and explained. • In pharmacokinetics the body is represented as a single or multiple compartments in to which the drug is distributed. • Some of the parameters are therefore a little abstract as we know the body is much more complicated ! Volume of Distribution, Clearance and Elimination Rate Constant V Volume 100 L Clearance 10 L/hr Volume of Distribution, Clearance and Elimination Rate Constant V V2 Cardiac and Skeletal Muscle Volume 100 L (Vi) Clearance 10 L/hr V2 Cardiac and Skeletal Muscle V Volume 100 L (Vi) Clearance 10 L/hr Volume of Distribution = Dose_______ Plasma Concentration V2 Cardiac and Skeletal Muscle V Volume 100 L (Vi) Clearance 10 L/hr Clearance = Volume of blood cleared of drug per unit time V2 Cardiac and Skeletal Muscle V Volume 100 L (Vi) Clearance 10 L/hr Clearance = 10 L/hr Volume of Distribution = 100 L What is the Elimination Rate Constant (k) ? CL = kV k = 10 Lhr -1 = 0.1 hr -1 100 L 10 % of the “Volume” is cleared (of drug) per hour k = Fraction of drug in the body removed per hour CL = kV If V increases then k must decrease as CL is constant Important Concepts • VD is a theoretical Volume and determines the loading dose • Clearance is a constant and determines the maintenance dose • CL = kVD • CL and VD are independent variables • k is a dependent variable Volume of Distribution Apparent volume of distribution is the theoretical volume that would have to be available for drug to disperse in if the concentration everywhere in the body were the same as that in the plasma or serum, the place where drug concentration sampling generally occurs. Volume of Distribution • An abstract concept • Gives information on HOW the drug is distributed in the body • Used to calculate a loading dose Loading Dose Dose = Cp(Target) x VD Question • What Is the is the loading dose required fro drug A if; • Target concentration is 10 mg/L • VD is 0.75 L/kg • Patients weight is 75 kg • Answer is on the next slide Answer: Loading Dose of Drug A • • • • • • Dose = Target Concentration x VD VD = 0.75 L/kg x 75 kg = 56.25 L Target Conc. = 10 mg/L Dose = 10 mg/L x 56.25 L = 565 mg This would probably be rounded to 560 or even 500 mg. Clearance • Ability of organs of elimination (e.g. kidney, liver to “clear” drug from the bloodstream • Volume of fluid which is completely cleared of drug per unit time • Units are in L/hr or L/hr/kg • Pharmacokinetic term used in determination of maintenance doses Maintenance Dose Calculation • Maintenance Dose = CL x CpSSav • CpSSav is the target average steady state drug concentration • The units of CL are in L/hr or L/hr/kg • Maintenance dose will be in mg/hr so for total daily dose will need multiplying by 24 Question • What maintenance dose is required for drug A if; • Target average SS concentration is 10 mg/L • CL of drug A is 0.015 L/kg/hr • Patient weighs 75 kg • Answer on next slide. Answer • Maintenance Dose = CL x CpSSav • CL = 0.015 L/hr/kg x 75 = 1.125 L/hr • Dose = 1.125 L/hr x 10 mg/L = 11.25 mg/hr • So will need 11.25 x 24 mg per day = 270 mg Half-Life and k • Half-life is the time taken for the drug concentration to fall to half its original value • The elimination rate constant (k) is the fraction of drug in the body which is removed per unit time. Drug Concentration C1 Exponential decay C2 dC/dt C = -k.C Time Log Concn. C0 C0/2 t1/2 t1/2 t1/2 Time Time to eliminate ~ 4 t1/2 Integrating: -kt .e Cp2 = Cp1 Logarithmic transform: lnC2= lnC1 - kt logC2 = logC1 - kt/2.303 Elimination Half-Life: t1/2 = ln2/k t1/2 = 0.693/k Steady-State • Steady-state occurs after a drug has been given for approximately five elimination half-lives. • At steady-state the rate of drug administration equals the rate of elimination and plasma concentration - time curves found after each dose should be approximately superimposable. Accumulation to Steady State 100 mg given every half-life 175 187.5 194 … 200 150 100 87.5 94 75 50 97 … 100 C Cpav t Four half lives to reach steady state What is Steady State (SS) ? Why is it important ? • Rate in = Rate Out • Reached in 4 – 5 half-lives (linear kinetics) • Important when interpreting drug concentrations in TDM or assessing clinical response Therapeutic Drug Monitoring Some Principles Therapeutic Index • Therapeutic index = toxic dose/effective dose • This is a measure of a drug’s safety – A large number = a wide margin of safety – A small number = a small margin of safety Drug Concentrations May Be Useful When There Is: An established relationship between concentration and response or toxicity A sensitive and specific assay An assay that is relatively easy to perform A narrow therapeutic range A need to enhance response/prevent toxicity Why Measure Drug Concentrations? • • • • Lack of therapeutic response Toxic effects evident Potential for non-compliance Variability in relationship of dose and concentration • Therapeutic/toxic actions not easily quantified by clinical endpoints Potential for Error When Using TDM • Assuming patient is at steady-state • Assuming patient is actually taking the drug as prescribed • Assuming patient is receiving drug as prescribed • Not knowing when the drug concentration was measured in relation to dose administration • Assuming the patient is static and that changes in condition don’t affect clearance • Not considering drug interactions