* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PPT here
Survey
Document related concepts
Discovery and development of proton pump inhibitors wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Transcript
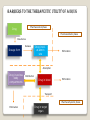
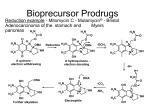
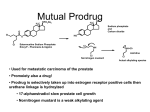
A seminar on Prodrugs BY K.SUNITHA M.Pharm. II SEMESTER DEPARTMENT OF INDUSTRIAL PHARMACY UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY WARANGAL ANDHRA PRADESH Contents: Introduction Various approaches to enhance efficacy of the drug Hard and soft drugs Prodrugs Classification of prodrugs Considerations in the design of prodrugs Strategies in the design of prodrugs Applications of prodrugs Prodrug based novel drug delivery approaches Conclusion References BARRIERS TO THE THERAPEUTIC UTILITY OF A DRUG Pharmaceutical phase Drug Pharmacokinetic phase Manufacture Release Dosage form Drug in sol. at admin site Elimination Absorption Drug in various body compartments Distribution Drug in blood Elimination Transport Pharmacodynamic phase Elimination Drug in target organ VARIOUS APPROACHES TO ENHANCE THE EFFICACY OF A DRUG: The therapeutic efficacy can be improved by minimizing or eliminating the undesirable properties while retaining the desirable ones. Biological approach Physical approach Chemical approach CHEMICAL MEANS OF OPTIMIZING THE DRUG THERAPEUTICS 1. Design and development of new drugs. 2. Design of hard and soft drugs. 3. Design of prodrugs. HARD DRUGS Resistant to biotransformation. Has a long half life. Design of hard drug involves metabolic stabilization. ADVANTAGES: Enhanced duration of action Less wastage of active moiety Avoids generation of potentially active harmful metabolites. Limits inter-subject variability Ex: Conversion of tolbutamide to chlorpropamide. SOFT DRUGS A soft drug is a biologically active compound that is biotransformed invivo in a rapid and predictable manner in to non toxic metabolites. Design of synthetic soft drug involves introduction of a group or a bond susceptible to rapid metabolic action. Ex: natural endogenous compounds such as adrenalin and insulin. Prodrugs Prodrugs are pharmacologically inactive derivatives of active drugs that are designed to maximize the amount of active drug that reaches its site of action, through manipulation of physicochemical, biopharmaceutical and pharmacokinetic properties of drug. They are converted into active drug within the body through enzymatic or nonenzymatic reactions. Also called drug latentiation. CLASSIFICATION OF PRODRUGS: 1.Carrier linked prodrugs: Active drug is covalently linked to an inert carrier or transporter moiety. They have enhanced lipophilicity due to attached carrier. The active drug is released by hydrolytic cleavage, either chemically or enzymatically. 2.Bioprecursors: These are inert molecules obtained by chemical modification of the drug but do not contain a carrier. Such a molecule has the same lipophilicity as the parent drug and is bioactivated generally by redox biotransformation, only enzymatically. Ex: NSAID – nabumetone (relafen) - arthritis. O CH3 CH3O Series of oxidative decarboxylation OH CH3O O Active form of the drug that inhibits Prostaglandin biosynthesis by cyclooxygenase 3. Mutual prodrugs: The prodrug comprises of two pharmacologically active agents coupled together to form a single molecule such that each acts as the carrier for the other. Ex: Benorylate is mutual prodrug of NSAIDs aspirin and paracetamol. Ex: Emcyt is a mutual prodrug containing estramustine and nornitrogen mustard linked to each other. H3C OPO3Na2 H3C OH Sodium phosphate and Carbon dioxide O Cl N Cl O Estermustine Sodium Phosphate Emcyt® - Pharmacia & Upjohn HO Cl NH NH+Cl- Aziridine Cl Nornitrogen mustard Cl Actual alkylating species Considerations in the design of prodrugs: 1. Purpose 2. Expected properties 3. Site of transformation 4. Mechanism of transformation 5. Chemical half life Strategies for the design of prodrugs: 1. Carriers: Carrier is an inert molecule or the promoiety attached to the active drug moiety through a metabolically labile linkage. The carrier imparts some desirable property to the drug such as increased lipid or water solubility. Carriers that help in directing the active moiety to the target site is called as specifier. Specifiers: Targeting unit part of the prodrug which directs the active moiety to the target site. Antibody Directed Enzyme Prodrug Therapy Gene Directed Enzyme Prodrug Therapy Polymer Directed Enzyme Prodrug Therapy/ Macromolecule Directed Enzyme Prodrug Therapy linkers: A releasable linker or spacer is incorporated between the specifier/carrier and the parent drug. Reasons for the application of linkers: 1. Incorporation of appropriate linkage between the promoiety and the active drug. 2. Facilitation of enzymatic action on carrier linked prodrugs. Classification of linkers: • • Electronic cascade linkers Cyclization linkers Doxorubicin attaches β-glucuronide was inert towards clevage by βglucuronidase. When they were linked by a 1,6-elimination linker, the substrate showed susceptability to the enzyme. simple spacers: Multiple release spacers: functional groups amenable to prodrug design Prodrug linkage and enzymes involved in the hydrolysis of linkage: Prodrug linkage Hydrolyzing enzymes Ester Short and medium chain Cholinesterases Aliphatic Ester hydrolase , Lipase , Cholesterol Esterase, Acetylcholinesterase , Aldehyde oxidase, Carboxypeptidase Long chain aliphatic carbonate Pancreatic lipase,Pancreatin,Lipase, Carboxypeptidase, Cholinesterase Phosphate, Organic Acid phosphatase, Alkaline Phosphatase Sulfate, organic Steroid sulfatase Prodrug Linkage Hydrolyzing Enzyme Amide Amidase Amino acid Proteolytic enzymes, Trypsin, Carboxypeptidase A and B, Azo Azo reductase Carbamate Carbamidase Phosphamide Phosphoramidases β-Glucuronide β-Glucuronidase N-Acetylglucosaminide α- N-Acetylglucosaminidase β-Glucoside β-Glucosidase APPLICATIONS OF PRODRUGS: 1.Pharmaceutical applications 2.Pharmacokinetic applications 3.Targeted drug delivery 4.Controlled drug delivery Pharmaceutical applications: 1.Improvement of taste: Bitter taste of the drug Unsuitable for preparation of suspension. Reduce the solubility of the drug in the saliva. PARENT DRUG PRODRUG WITH IMPROVED TASTE chloramphenicol Palmitate ester clindamycin Palmitate ester sulfisoxazole Diacetate ester O O 2N CH CH CH 2OC (CH 2)14CH 3 CH3 NH Lipase O 2N CH CH CH3 NH O CH3 Chloramphenicol palmitate CH 2 OH O CHCl 2 chloramphenicol 2. Improvement of odor: •The odor of the compound depends upon its vapor pressure. Ex: ethyl mercaptan is a foul smelling liquid of b.p. 35ᴼC. It is converted into its phthalate ester, diethyldithio-isophthalate ester which is odorless and has higher boiling point. O C SC2H5 C SC2H5 O Diethyldithio-isophthalate thioesterase CH3CH2SH ethyl mercaptan 3. Change of physical form of the drug: •Liquid form of the drug unsuitable to formulate as a tablet. •Conversion of liquid drugs to solid prodrug involves the formation of symmetrical molecules having a tendency to crystallize. Ex: 1,3-diester derivative of ethyl mercaptan. 4. Reduction of gastric irritation: • Increased stimulation of acid secretion. • Interference with the protective mucosal layer. parent drug prodrugs Salicylic acid Aspirin Diethyl stilbestrol Fosfestrol Phenyl butazone N-methyl piperazine salt Oleandrin Oleandrine acetate 5. Reduction of pain on injection • Drug precipitates or penetrates into the surrounding tissues. • The solution is strongly acidic, alkaline or alcoholic. Clindamycin-2’-phosphate ester Phosphatase Clindamycin HCl 6.Enhancement of solubility and dissolution rate: Dissolution is the rate limiting step in the absorption of drug. Parenteral and ophthalmic formulations of such agents required. Parent drug Prodrugs with enhanced hydrophilicity Chloramphenicol Sodium succinate ester Tocopherols Sodium succinate ester Testosterone Phosphate ester Diazepam L-lysine ester 7. Enhancement of chemical stability: Drug must be stable during its shelf-life. Penicillins susceptible to hydrolysis and destabilization in acidic pH of stomach. Parent drug Prodrugs with enhanced stability Azacytidine Azacytidine bisulfite carbenicillin Carindacillin (α-indanol ester) Carfecillin (α-phenyl ester) erythromycin Stearate, ethyl succinate and estolate prodrugs Azacytidine bisulfite pH = 7.4 II. Pharmacokinetic applications: 1. Increasing the bioavailability: Lipophilic drugs passively absorbed Reasons: (a) Lipophilic form of drug has enhanced membrane /water partition coefficient as compared to hydrophilic form of drug. Ex: pivampicillin and bacampicillin prodrugs of ampicillin are more lipophilic, better absorbed (above 98%) and rapidly hydrolyzed to the parent drug in blood. (b) The lipophilic prodrugs of some drugs have poor solubility in gastric fluids and thus greater stability and better absorption. Ex: esters of erythromycin. Advantage: dose reduction Ex: Pivampicillin is as effective as Ampicillin in just one third of the dose of the latter. 2. Prevention of presystemic metabolism Ester and ether prodrugs of corticosteroids bypass presystemic metabolism. Ex: triamcolone acetonide Propronalol has high first pass metabolic effect. Its hemisuccinate ester is resistant to esterases of liver. 3. Reduction of toxicity: NSAIDs cause gastric distress Timolol and Epinephrine in the treatment of glaucoma. Lipophilic ester prodrugs of these drugs have a better intraocular penetration enabling a reduction in dose and thus adverse effects are minimised. • the therapeutic index of alkyl ester prodrugs of timolol increased by 16times. • the therapeutic index of a diester of epinephrine with pivalic acid increased by 10times. The epinephrine prodrug also has increased resistance to oxidation. 4. Prolongation of duration of action: The two rate controlling steps in the enhancement of duration of action are: (i) The rate of release of prodrug from the site of application (ii) The rate of conversion of prodrug into active drug Ex: i.m. depot injections of lipophilic ester prodrugs of steroids (testosterone cypionate) esters of antipsychotics (fluphenazine enanthate and decanoate) Bambuterol is a biscarbonate ester prodrug of β2-antagonist terbutaline in the treatment of asthama. Slowly converted into active form by hydrolysis by cholineaterases. It bypasses presystemic elimination. Maximum plasma concentration of terbutaline occurs approxiamately 4-7hrs after bambuterol administration and efficacy lasts for 24hrs. Therefore dose is reduced from thrice a day to once a day. Requirements for Prodrugs designed for targeted therapy: 1. The tissue associated biomolecule must be present in significantly elevated levels in that particular tissue compared to normal tissue. 2. Prodrug level must be high enough to generate therapeutic levels of free drug in the target tissue. 3. Prodrug activation at the other sites must be minimal. 4. Prodrugs must be good substrate or possess high binding affinity for tissue associated molecule. 5. It must not be rapidly eliminated from the body and must not enter cells randomly. Strategies for targeting prodrugs 1. Prodrug monotherapy: These are designed for direct activation or recognition by a tumor/tissue associated factor. Is a one-step therapy, only the prodrug is administered. 2. Two step prodrug therapy: Enzyme which can activate the prodrug is administered in the first step. In the second step prodrug is administered. ADEPT GDEPT - VDEPT/BDEPT PDEPT/ MDEPT Assessment of efficiency of drug targeting Maximum tolerated dose Therapeutic index (T.I.) = Minimum effective dose T.I. of targeted drug Therapeutic advantage = T.I. of non-targeted drug Drug targeting index = (AUCtarget site/AUCtoxic site )target drug (AUCtarget site/AUCtoxic site)non-targeted drug Tumor targeting: Most chemotherapeutic agents are highly toxic with narrow therapeutic index. They also effect normal dividing cells. Undesirable side effects- nausea, vomiting, hair loss, diarrhea, etc. Development of multi drug resistance by the tumor cells. Hypoxic cells resistant to radiotherapy. Irregular blood flow leads to reduced supply of glucose and other nutrients to the cancer cells, slowing down their rate of division. Thus it becomes difficult to target these cells with chemotherapeutic agents that kill fast multiplying cells. Tumor associated factors for targeting: 1. Hypoxia: The hypoxic condition of tumor tissue is exploited in targeting. Hypoxia selective cytotoxic agents used in combination therapy with conventional anticancer chemotherapeutic agents. Hypoxia selective drugs kill hypoxic tumor cells and other agents kill aerobic tumor cells. Ex: N-oxide bioreductive tirapazamine (phase-III clinical trials) is used to treat lung cancer along with cisplatin. Tumor associated factors and prodrugs Protease Prodrugs Cathepsin-B Dipeptides of Daunorubicin, Paclitaxel, Mitomycin C Plasmin and u-PA system Peptide linked Nitrogen mustards Matrix metalloproteinases 2-L-pyroglutamyl-MTX Serine protease (prostate specific) Heptapeptide Dauxorubicin Receptors Prodrugs Folate receptors Daunorubicin-β melanocyte stimulating hormone Hyaluronic acid receptors Hyaluronic acid-paclitaxel Asialoglycoprotein receptors Galactose amine - DOX Prodrugs for ocular delivery: Tight corneal epithelium Precorneal drug elimination Systemic drug absorption Less than 1% of the instilled drug reaches the intra ocular tissue. Ocular absorption of drugs can be substantially enhanced by increasing lipophilicity and solubility of the drug . Prodrugs for brain targeting: BBB is a well-organized dynamic interface that actively and selectively regulates both the uptake of molecules from the blood into the brain and efflux from the brain parenchyma back into the systemic circulation. Conversion into lipophilic form leads into simultaneous enhancement in transport of drug to other tissues there by greatly increasing the chance of systemic toxicity. Dihydropyridine-pyridinium salt redox system: The drug to be delivered to brain is covalently linked to the lipophilic dihydropyridine carrier to form a prodrug which partitions across the BBB. The reduced or the dihydro carrier is oxidized by NAD-NADH system. Ex: Another inert carrier used to target drugs to brain is dihydro trigonelline. It can be linked to dopamine and targeted to brain. Ex: N-methyl pyridinium-2-carbaldoxime (2-PAM or Pralidoxime), a cholineaterase reactivator. Prodrugs for liver targeting Receptors Asilogycoprotein (AGP), LDL,HDL, insulin, EGF, transferrin Transporters NCTP, OATs, OCTs enzymes Many enymes, CYP3A4 Targeting CYP3A4: Adefovir bispivaloyl oxymethyl prodrug of 9-(2-phosphonylmethoxyethyl)adenine [PMEA], an antiviral drug. It is activated into active drug by esterases. Pradefovir is hepdirected prodrug of PMEA. Asialoglycoprotein receptors: AGP receptors recognize asialofeutin, asialoorosomucoid, albumin and lipoproteins that are randomly derivatized with sugar groups and other polymers. Drugs: Ribavirin, MTX, Dox, Daunorubicin, Primaquine Recent advances in prodrug targeting: ADEPT: Antibody is directed at a tumor-associated antigen to convey an enzyme to tumor sites and, from a low toxic prodrug a high toxicity drug would be released by enzymatic catalysis within tumors. The active drug would be a small molecule able to diffuse within a tumor The free enzyme circulating in the blood is removed by using galactosylated murine derived antibodies that are recognized and removed by hepatic cells. Monoclonal antibody Trade name Manufacturer target cancer bevacizumab Avastin genentech VEGF Colon cancer Cetuximab Erbitux Merck EGF Colorectal, head and neck Pantitumumab Vactibix Amgen EGF Colorectal pertuzumab omniteag Gentech HER2 Breast cancer Viral vectors: Adeno virus, adeno associated virus, lenti virus, retrovirus, herpes virus, etc.. Purine nucleotide phosphorylase/fludarabine system in the treatment of prostate cancer (entered into phase I clinical trails), targeted using Rous sarcoma virus. Poymeric prodrugs Prodrugs in novel drug delivery systems: 1.Prodrugs in liposomes: The triamcolone 21-palmitate showed 85% entrapment efficiency compared to triamcolone acetonide (5%). The 6-mercaptopurine showed EE of only 1.5%. When it is linked to glyceryl monostearate its EE was increased to 98%. 5-Flurouridine (FUR) showed low (26%) EE and rapid leakage during storage (>50% in 12days). The lipophilic 5’-palmitoyl-5-FUR showed 95% EE and no leakage during storage. 2. Prodrugs in solid lipid nanoparticles: Incorporation of prodrug in the triglyceride core releases drug slowly. Ex: the incorporation of azidothymidine (AZT) in SLN was minimal (<1%) but the incorporation of AZT-palmitate ester prodrug increased with increasing phospholipid content to the maximum of 90%. 3. Prodrugs in niosomes: Niosomes are vesicles of non ionic surfactant. Chemically more stable than the phospholipids. Ex: N-(2-hydroxypropyl)methacraylamide – doxorubicin prodrug showed four-fold increase in maximum tolerated dose and anti-tumor activity (phase III). Conclusion Recent advances in biotechnology have made it possible to utilize pro-drug design to develop site specific drug delivery systems which provide various means of targeting the delivery of parent drugs to specific sites within the body. Clearly, the increasing demands for more efficacious and less toxic drugs will ensure that prodrug approaches continue to be exploited in the development of future drug substances. References: •V.J. Stella, R.T. Borchardt, M.J. Hageman, R. Oliyai, H. Maag, J.W. Tilley, Prodrugs: Challenges and Rewards, Parts 1 and 2 Volume V: •John H. Block and John M. beale, Jr. ,Wilson and Gisvold’s textbook of Organic medicinal and Pharmaceutical chemistry, eleventh edition, pg: 142-155. •Biopharmaceutics and Pharmacokinetics by D.M.Brahmankar and Sunil B.Jaiswal, pg: 159-176. •Advances in Controlled and Novel Drug Delivery by N.K.Jain, pg: 269-285. •Xiaoling Li, Ph.D, Bhaskara R. Jasti, Ph.D, Design of Controlled Release Drug Delivery Systems, pg: 75-105. •Binghe Wang, terune Siahaan, and Richard A. Soltero’s Drug delivery principles and applications •Review article on Exploitation of Bile Acid Transport Systems in Prodrug Design by Elina Sievanen University of Jyvaskyla, Department of Chemistry,University of Jyvaskyla, Finland 14 August 2007 / Published: 16 August 2007