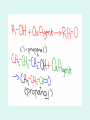* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download aldehydes powerpoint
Survey
Document related concepts
Fischer–Tropsch process wikipedia , lookup
Marcus theory wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Metal carbonyl wikipedia , lookup
Elias James Corey wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Ene reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Discodermolide wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Aldol reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Transcript
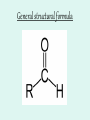
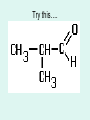
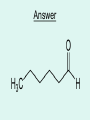
Aldehydes By: Nicole Murphy & Michaela Miller General Structure • What makes an aldehyde an aldehyde? A carbon double bonded to an oxygen molecule. This is called a carbonyl group. Bonded to this carbonyl group is a hydrogen molecule. General structural formula Naming Aldehydes are named using the IUPAC rules. • 1) Identify the longest carbon chain with the carbonyl group. Naming cont. 2) When numbering, the carbonyl group is always number 1. 3) Identify the branched attachments (alphabetically) and prefix the carbon number it is attached to. If there is more than one of the same type use prefixes. Ex: di for 2, tri for 3 ect. Naming cont. 4) Use the alkane name that represents the number of carbons in the longest chain. 5) Change the “e” ending and replace it with “al”. Try this…. Answer…. • 2-methylpropanal Try this… •Draw: - Hexanal Answer Everyday use: •Hexanal is actually used in the flavour industry to produce fruity flavours, although its scent resembles that of freshly cut grass. • Smaller Aldehydes have a stronger, sharp, pungent odour. Larger Aldehydes have an almost rosy smell, or a sweet smell. • Formaldehyde (or CH2O) can be used to preserve dead animals. • Acetone is commonly found in fingernail polish remover and is a solvent. • 2-Butanone is used as a solvent and paint stripper. •Aldehydes are famously produced as ingredients in perfumes and flavors. • Carvone is used as spearmint flavoring. • Vanillin is the vanilla flavoring and gives vanilla beans their aroma. (Often appears in nature) • Cinnamaldehyde smells like cinnamon. Properties of Aldehydes • Aldehydes have a higher boiling point than Alkanes of similar size and structure. • Ex. Boiling point of 2-methylpropanal : 63˚C vs. Boiling point of 2methylpropane: 11.7 ˚C • Aldehydes with lower molecular weight have high water solubility (ex. Formaldehyde). Common reactions • Aldehydes are formed by partial oxidation of primary alcohols and form carboxylic acids when they are further oxidized. • Two common aldehyde reactions are either the reduction or oxidation reaction. When an oxidizing agent is added to a primary alcohol it forms an aldehyde, or if an aldehyde is reduced, it can form a primary alcohol. REACTION Synthesis: A primary alcohol reacts with an oxidizing agent to make an aldehyde R-OH + Ox.Agent RH= O Example: 1-Propanol is oxidized to propanal CH3CH2CH2OH + Ox.Agent CH3CH2CHO Examples of Oxidizing Agents An oxidizing agent is the substance that provides oxygen or removes hydrogen from another substance. • Permanganate (MnO4-) • When Hydrogen gas reacts with metals it is an oxidizing agent. (H2(g)) • Oxygen gas (O2 ) Another Oxidation Reaction • Tollens' reagent is a chemical reagent most commonly used to determine whether a known carbonyl-containing compound is an aldehyde or a ketone. It is usually ammoniacal silver nitrate, but can also be other mixtures, as long as aqueous diamminesilver(I) complex is present. It was named after its discoverer, Bernhard Tollens. • A positive test with Tollens' reagent results in elemental silver precipitating out of solution, occasionally onto the inner surface of the reaction vessel, producing a characteristic and memorable "silver mirror" on the inner vessel surface.