* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Cracking (chemistry) wikipedia , lookup
Aromaticity wikipedia , lookup
Discodermolide wikipedia , lookup
Homoaromaticity wikipedia , lookup
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Persistent carbene wikipedia , lookup
Hydroformylation wikipedia , lookup
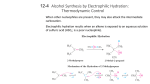
12-7 Oxymercuration-Demercuration: A Special Electrophilic Addition The electrophilic addition of a mercuric salt to an alkene is called mercuration. The product formed is known as an alkylmercury derivative. A reaction sequence known as “oxymercuration-demercuration” is a useful alternative to acid-catalyzed hydration: Oxymercuration is anti stereospecific and regioselective. The alcohol obtained from oxymercuration-demercuration is the same as that obtained from Markovnikov hydration, however, since no carbocation is involved in the reaction mechanism, rearrangements of the transition state do not occur. Oxymercuration-demercuration in an alcohol solvent yields an ether: 12-8 Hydroboration-Oxidation: A Stereospecific Anti- Markovnikov Hydration The boron-hydrogen bond adds across double bonds. Borane, BH3, adds to double bonds without catalytic activation: The borane is commercially available in an ethertetrahydrofuran solvent. Hydroboration is regioselective as well as stereospecific (syn addition). Here, steric factors are more important than electronic factors. The boron binds to the less hindered (substituted) carbon. The oxidation of alkylboranes gives alcohols. The oxidation of a trialkylborane by hydrogen peroxide produces an alcohol in which the hydroxyl group has replaced the boron atom. In this reaction, the hydroxyl group ends up at the less substituted carbon: an antiMarkovnikov addition. 12-9 Diazomethane, Carbenes and Cyclopropane Synthesis Cyclopropanes can be readily prepared by the addition of a carbene to the double bond of an alkene. A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electron rich alkene. Diazomethane forms methylene, which converts alkenes into cyclopropanes. The highly reactive species methylene, H2C: (the simplest carbene) can be produced from the decomposition of diazomethane: When methylene is generated in the presence of an alkene, an addition reaction occurs producing a cyclopropane. This reaction is usually stereospecific, with retention of the original double bond configuration. Halogenated carbenes and carbenoids also give cyclepropanes. Halogenated carbenes, prepared from halomethanes, can also be used to synthesize cyclopropanes. Treatment of trichloromethane (chloroform) with strong base causes an elimination reaction in which both a proton and a chlorine atom are removed from the same carbon. The resulting product is a dichlorocarbene which reacts with alkenes to produce cyclopropanes. To avoid the hazards associated with diazomethane preparation, an alternate route using diiodomethane and zinc (Simmons-Smith reagent) to produce ICH2ZnI is used. This substance is an example of a carbenoid, a carbenelike substance that converts alkenes into cyclopropanes stereospecifically.