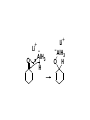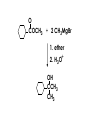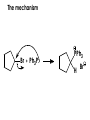* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 17. Aldehydes and Ketones
Survey
Document related concepts
Aromaticity wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Elias James Corey wikipedia , lookup
Discodermolide wikipedia , lookup
Homoaromaticity wikipedia , lookup
Kinetic resolution wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Aldol reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Asymmetric induction wikipedia , lookup
Transcript
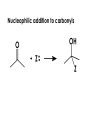
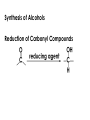
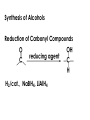
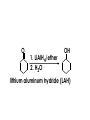
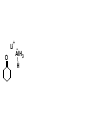
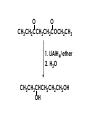
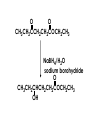
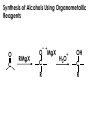
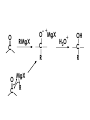
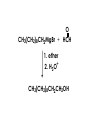
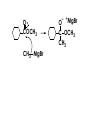
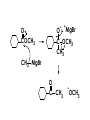
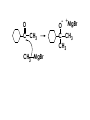
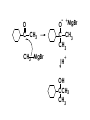
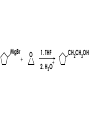
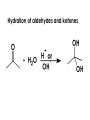
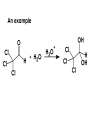
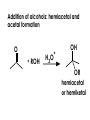
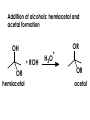
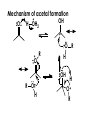
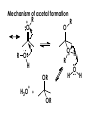
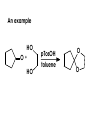
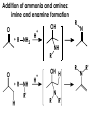
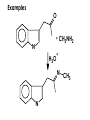
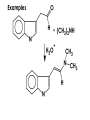
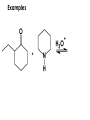
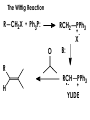
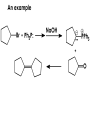
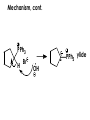
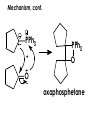
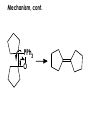
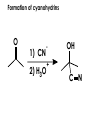
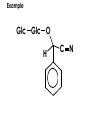
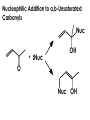
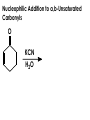
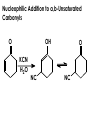
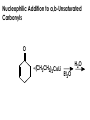
Aldehydes and Ketones carbonyl compounds carbonyl group structure, bonding and physical properties Review of methods of preparation of aldehydes and ketones hydration of alkynes Friedel-Crafts acylation of aromatic compounds Overview of reactions of carbonyl compounds reduction to alcohols addition of organometallic reagents addition of oxygen, nitrogen, sulfur and phosphorus nucleophiles reduction of carbonyl to methylene (Clemmensen or Wolff-Kishner) Nucleophilic addition to carbonyls Nucleophilic addition to carbonyls Synthesis of Alcohols Reduction of Carbonyl Compounds Synthesis of Alcohols Reduction of Carbonyl Compounds Synthesis of Alcohols Reduction of Carbonyl Compounds H2/cat., NaBH4, LiAlH4 O 1. LiAlH 4/ether 2. H2O OH lithium aluminum hydride (LAH) O + Li AlH3 H O + Li AlH3 H Li + -AlH3 O H O O CH3CH2CCH2CH2COCH2CH3 1. LiAlH 4/ether 2. H2O CH3CH2CHCH2CH2CH2OH OH O O CH3CH2CCH2CH2COCH2CH3 NaBH4/H2O sodium borohydride O CH3CH2CHCH2CH2COCH2CH3 OH Synthesis of Alcohols Using Organometallic Reagents O C RMgX + O MgX C R + H3O OH C R O C RMgX + O MgX C R O C MgX R + H3O OH C R O CH3(CH2)8CH2MgBr + HCH 1. ether + 2. H O 3 CH3(CH2)8CH2CH2OH O COCH3 + 2 CH3MgBr 1. ether + 2. H O 3 OH CCH3 CH3 O COCH3 CH3 MgBr O +MgBr C OCH3 CH3 O COCH3 O +MgBr C OCH3 CH3 CH3 MgBr O C CH3 -OCH 3 O C CH3 CH3 MgBr O +MgBr C CH3 CH3 O C CH3 CH3 MgBr O +MgBr C CH3 CH3 + H OH CCH3 CH3 MgBr + O 1. THF + 2. H3O CH2CH2OH Hydration of aldehydes and ketones An example Addition of alcohols: hemiacetal and acetal formation Addition of alcohols: hemiacetal and acetal formation O + + ROH OH H3O OR hemiacetal or hemiketal Addition of alcohols: hemiacetal and acetal formation OH + + OR hemiacetal ROH OR H3O OR acetal Mechanism of acetal formation O + H OH2 OH + + O R O R H OH R O H H O+ R Mechanism of acetal formation + R R O O R O R H OR + H3O + OR O+ H H O H An example HO O + HO pTosOH toluene O O Addition of ammonia and amines: imine and enamine formation O + + R NH2 OH H R NH R N Examples O + N CH3NH2 + H3O N CH 3 N Examples Examples The Wittig Reaction R CH2X + Ph3P: RCH2 PPh3 + - X O R H B: RCH PPh3 + YLIDE An example The mechanism Br + Ph3P: PPh3 H Br Mechanism, cont. PPh3 H Br PPh3 ylide OH Mechanism, cont. PPh3 + PPh3 O O oxaphosphetane Mechanism, cont. PPh3 O Formation of cyanohydrins O 1) CN - OH + 2) H3O C N Example Glc Glc O H C N Reduction of carbonyls to alkanes Clemmensen reduction Wolff-Kishner reduction Desulfurization of thioketals Nucleophilic Addition to a,b-Unsaturated Carbonyls Nuc + Nuc OH O Nuc OH Nucleophilic Addition to a,b-Unsaturated Carbonyls Strong nucleophiles add 1,2 kinetic control Weaker nucleophiles add 1,4 thermodynamic control Nucleophilic Addition to a,b-Unsaturated Carbonyls O KCN H2O Nucleophilic Addition to a,b-Unsaturated Carbonyls O OH KCN H2O NC O NC Nucleophilic Addition to a,b-Unsaturated Carbonyls O + (CH3CH2) CuLi 2 H2O Et2O Nucleophilic Addition to a,b-Unsaturated Carbonyls O O + (CH3CH2)2CuLi H2O Et2O CH3CH2