* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chem 150 Unit 2 - Hydrocarbons & Functional Groups
Bent's rule wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
History of chemistry wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Catalytic reforming wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Atomic theory wikipedia , lookup
Computational chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Cation–pi interaction wikipedia , lookup
Biological aspects of fluorine wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Hydrogen bond wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Chemical bond wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Homoaromaticity wikipedia , lookup
Acid strength wikipedia , lookup
Hydroformylation wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Abiogenesis wikipedia , lookup
History of molecular theory wikipedia , lookup
Aromatization wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Organic chemistry wikipedia , lookup
Aromaticity wikipedia , lookup
Chem 150
Unit 2 - Hydrocarbons &
Functional Groups
Organic chemistry is the chemistry of carbon. The name
“organic” reflect the fact that organic molecules are
derived from living organisms. In this unit will start by
looking at four families of organic molecules that are
grouped together as the hydrocarbons. We will also look
at some functional groups that define some of the other
families of organic molecules.
Organic Chemistry
Organic chemistry is the chemistry of carbon.
• There are three forms of pure carbon
2
•
Diamond
•
Graphite
Organic Chemistry
Organic chemistry is the chemistry of carbon.
• There are three forms of pure carbon
•
3
Buckminsterfullerene
“Bucky Balls”
Hydrocarbons
•
Organic molecules contain carbon combined with other
elements.
•
Organic molecules are grouped into families
• Members of a family share common structural, physical, and chemical
characteristics.
4
•
There are four families that contain molecules made of only
carbon and hydrogen.
•
Hydrocarbons
•
Alkanes
•
Alkenes
•
Alkynes
•
Aromatics
Hydrocarbons
5
Alkanes
Alkanes are hydrocarbons that contain only carbon-carbon
single bonds.
• Every carbon atom participates in 4 single bonds, either to
another carbon or to a hydrogen.
• Every hydrogen atom is bonded to a carbon by a single
bond.
6
Alkanes
Alkanes are hydrocarbons that contain only carbon-carbon
single bonds.
7
Alkanes
•
•
Alkanes in which the carbons are connected in a straight
chain are called normal alkanes.
H
H
H
H
H
H
H C
C
C
C
C
C
H
H
H
H
H
H
H
n-hexane
Alkanes that are branched are called branched chain
alkanes.
H
H
C
H
8
H
H
H
H
H C
C
C
C
C
H
H
H
H
H
2-methyl-pentane
H
Alkanes
For a discusion on the structure of alkanes,
see the Unit 2
Elaboration - Alkane Structure
Alkanes
10
•
Alkanes, along with the other hydrocarbons, are non-polar.
•
They interact with each other only through London
dispersion forces.
•
This is why they have relatively low boiling and melting
points.
Alkanes
They interact with each other only through London dispersion
forces.
• Note how the boiling points increase with molecular weight.
11
Molecule in the News
12
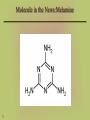
Molecule in the News:Melamine
13
Organic Molecules in the News!!
http://www.cbc.ca/health/story/2007/09/06/additives-lancet.html?ref=rss
http://www.medpagetoday.com/Psychiatry/ADHD-ADD/tb/6610
Quinoline yellow
Carmoisine
14
Sodium benzoate
Alkanes
Alkanes, cannot be named based on their molecular formulas
• For example, all of the molecules shown below share the
same molecular formula, C6H14
(hexacarbon tetradecahydride?)
H
H
H
H
H
H
H C
C
C
C
C
C
H
H
H
H
H
H
H
n-hexane
H
H
C
H
H
H
H
H
H
H
H C
C
C
C
C
H
H
H
H
H
H
H
H
H C
C
H
H
C
H
H
H
H
H
H
C
C
C
H
H
H
C
H
H
H C
C
H
H
C
H
H
H
H
C
C
H
H
H
H
H
2-methyl-pentane
15
3-methyl-pentane
2,2-dimethylbutane
H
H
H C
C
C
H
H
H
C
H
C
C
H
H
H
H
H
2,3-dimethylbutane
Alkanes
Organic chemists use a systematic set of rules, called the
IUPAC rules, to name organic molecules based on their
structural formulas instead of their chemical formulas.
H
H
H
H
H
H
H C
C
C
C
C
C
H
H
H
H
H
H
H
n-hexane
H
H
C
H
H
H
H
H
H
H
H C
C
C
C
C
H
H
H
H
H
H
H
H
H C
C
H
H
C
H
H
H
H
H
H
C
C
C
H
H
H
C
H
H
H C
C
H
H
C
H
H
H
H
C
C
H
H
H
H
H
2-methyl-pentane
16
3-methyl-pentane
2,2-dimethylbutane
H
H
H C
C
C
H
H
H
C
H
C
C
H
H
H
H
H
2,3-dimethylbutane
Alkanes
For a discussion on naming alkanes,
see the Unit 2
Elaboration - Naming Alkanes
Constitutional Isomers
When two or more molecules share the same molecular
formula, but have different atomic connections, they are
called constitutional isomers.
H
H
H
H
H
H
H C
C
C
C
C
C
H
H
H
H
H
H
H
n-hexane
H
H
C
H
H
H
H
H
H
H
H C
C
C
C
C
H
H
H
H
H
H
H
H
H C
C
H
H
C
H
H
H
H
H
H
C
C
C
H
H
H
C
H
H
H C
C
H
H
C
H
H
H
H
C
C
H
H
H
H
H
2-methyl-pentane
18
3-methyl-pentane
2,2-dimethylbutane
H
H
H C
C
C
H
H
H
C
H
C
C
H
H
H
H
H
2,3-dimethylbutane
Question (Clicker)
Which of the following is a constitutional isomer of this
molecule:
CH3
H3C
CH CH CH2 CH2 CH3
CH3
CH3
A)
H3C
CH CH CH2 CH3
C)
CH3
H2C
CH3
CH
CH CH2
CH3 CH3
CH3
H
H
B)
CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH3
D)
H
H
H C
C
C
H
H
19
H
C
H
H
H
C
C
H
H
H
Conformations
Carbon-carbon single bonds are free to rotate
20
•
This leads to different shapes for some molecules
•
These should not be confused with isomers.
Conformations
All of the 3-dimensional models shown below are for the nbutane.
• They were generated by rotating the central carbon-carbon
bond.
• They all share the same structural formula.
H
21
H
H
H
H
C
C
C
C
H
H
H
H
H
Conformations
All of the 3-dimensional models shown below are for the nbutane.
• They were generated by rotating the central carbon-carbon
bond.
22
Conformations
Switching from one conformation to another does not require
the breaking and making of covalent bonds.
•
Switching from one isomer to another does require the
breaking and making of covalent bonds.
n-butane
2-methylpropane
H
H
H
H
H
H
C
C
C
C
H
H
H
H
H
23
H
H
H
C
H
H
C
C
C
H
H
H
H
Conformations
For a discussion on conformations,
see the Unit 2
Elaboration - Conformations
Question (Clicker)
True or False? Constitutional isomers have the same IUPAC
name.
• True
• False
25
Question (Clicker)
True or False? The different conformations of an alkane have
the same IUPAC name.
• True
• False
26
Cycloalkanes
When there are three or more carbons in a straight chain, the
ends can be joined to make rings.
27
•
In naming these molecules, the prefix cyclo- is used to
indicate the ring:
•
Skeletal structural formulas are used to represent the rings
in structural formulas:
Cycloalkanes
In naming these molecules, the prefix cyclo- is used to
indicate the ring:
As Parent Chain
As Substituent Group
C 3H 6
cyclopropane
R
cyclopropyl-
cyclobutane
R
cyclobutyl-
R
cyclopentyl-
C 4H 8
C5H10
cyclopentane
C6H12
cyclohexane
28
R
cyclohexyl-
Cycloalkanes
The carbon-carbon single bonds for the carbons in a ring are
no longer free to rotate.
•
•
This leads to a new type of isomer
Since the two structures share the same name, they are
not constitutional isomers.
CH3
CH3
CH3
1,2-dimethylcyclohexane
H
H
H
H
29
H
CH3
H
H
H
H
CH3
H
H
CH3
1,2-dimethylcyclohexane
H
H
H
CH3
H
H
H
H
H
CH3
Cycloalkanes
Isomers which share the same atomic connections, and
therefore, the same IUPAC name are called stereoisomers.
•
•
When this occurs due to restricted rotation about a
covalent bond, they are called geometric isomers
The prefix cis- and trans- are used to distinguish geometric
isomers.
CH3
CH3
CH3
cis-1,2-dimethylcyclohexane
H
H
H
H
30
H
CH3
H
H
H
H
CH3
H
H
CH3
trans-1,2-dimethylcyclohexane
H
H
H
CH3
H
H
H
H
H
CH3
Questions
Draw the condensed structural formulas for the following
molecules:
A)
1-ethyl-2-methylcyclopentane
B)
1,1-dimethylcyclobutane
C)
1,1-dimethyl-2-propylcyclopropane
Do any of these molecules have cis- and trans- geometric
isomers?
31
Alkenes, Alkynes & Aromatic Compounds
The remaining three families of hydrocarbons are
unsaturated.
•
Alkanes are saturated, which means they contain the
maximum number of hydrogens per carbon.
•
•
Alkenes, Alkynes and Aromatics are unsaturated, which
means they contain less than the maximum number of
hydrogens per carbon.
•
32
For alkanes CnH(2n+2)
Structurally, this means that they have carbon-carbon double or triple bonds
Alkenes, Alkynes & Aromatic Compounds
Alkenes are hydrocarbons that contain at least 1 carboncarbon double bond.
• Examples:
H
H
C
H
C
H
C
H
ethene
(ethylene)
33
H
H
C
CH2 CH2 CH2 CH3
1-hexene
Alkenes, Alkynes & Aromatic Compounds
Alkynes are hydrocarbons that contain at least 1 carboncarbon triple bond.
• Examples:
H
C
C
H
ethyne
(acetylene)
34
H
C
C CH2 CH2 CH2 CH3
1-hexyne
Alkenes, Alkynes & Aromatic Compounds
Aromatics are unsaturated ring molecules
• They are often drawn to look like alkenes, but they behave
much differently than alkenes.
• They have an alternating pattern of double and single
bonds within a ring.
• Benzene is an example
35
Alkenes, Alkynes & Aromatic Compounds
The physical properties of all hydrocarbons are the same
•
The have essentially one noncovalent interaction, which
isthe London dispersion force.
•
They have no electronegative atoms and therefore have
•
No ion/ion interactions
• No dipole/dipole interactions
•
36
No hydrogenbonding interactions
Alkenes, Alkynes & Aromatic Compounds
Naming of Alkenes and Alkynes work the same as for
alkanes, with these added rules:
•
The parent chain must include both carbons in all double
and triple bonds.
•
•
The -ene ending is used of alkenes
•
The -yne ending is used for alkynes.
•
The number of the first carbon in the double or triple bond is
included in the name to locate the double or triple bond.
•
37
Pick the longest chain that also contains all double and triple bonds
Number the parent chain from the end that is closes to the first double or triple
bond.
Alkenes, Alkynes & Aromatic Compounds
Naming of Aromatics is based on benzene:
• When the molecule is build on benzene, the parent name
is “benzene”.
• There are also many common names used to describe
aromatic compounds.
38
Alkenes, Alkynes & Aromatic Compounds
Naming of Aromatics is based on benzene:
• Aromatic compounds can contain multiple aromatic rings
39
Alkenes, Alkynes & Aromatic Compounds
Benzo(a)pyrene found in tobacco smoke is converted to
carcinogenic products in the liver (see below) which link to
DNA and cause mutations.
40
Practice Quiz 1 KEY
http://www.chem.uwec.edu/Chem150_S07/course/answers/C
150-Quiz-1-key.swf
41
Alkenes, Alkynes & Aromatic Compounds
There are many aromatic molecules found in biology
• Some aromatic compounds contain nitrogen and oxygen
atoms
• For example, the nucleotide base Adenine, which is used
to make DNA and RNA
NH2
N
N
N
42
Alkenes, Alkynes & Aromatic Compounds
Like cycloalkanes, some alkenes can have cis and trans
isomers
•
•
This is due to restricted rotation about the double-bond.
Not all double bonds produce cis and trans isomers
•
Each carbon participating in the double bond must have two different
substituents attached to them
A
X
C
B
C
Y
A ≠ B AND X ≠ Y
43
Alkenes, Alkynes & Aromatic Compounds
Like cycloalkanes, some alkenes can have cis and trans
isomers
44
Alcohols, Carboxylic Acids & Esters
In addition to the four families of hydrocarbons, there are also
many other families of organic molecules.
These other families include elements other than carbon and
hydrogen.
45
•
They exhibit a wide range of chemical and physical
properties.
•
The families are distinguished by a group of atoms called a
functional group
Alcohols, Carboxylic Acids & Esters
Functional Group
“A functional group is an atom, group of atoms or bond that
gives a molecule a particular set of chemical and physical
properties”
46
Alcohols, Carboxylic Acids & Esters
The carbon-carbon double bonds found in alkenes is an
example of a functional group.
•
47
A chemical property of a double is that it will absorb
hydrogen in the hydrogenation reaction.
Alcohols, Carboxylic Acids & Esters
We look now at three families that are distinguished by a
functional group that contains the element oxygen.
Alcohols
• Members of the alcohol family contain a hydroxyl group.
• The hydroxyl group comprises an oxygen with one single
bond to a hydrogen and another single bond to an alkanetype carbon
H
H
H
C
C
H
H
O
An alkane-type carbon atom
ethanol
48
H hydroxyl group
Alcohols, Carboxylic Acids & Esters
We look now at three families that are distinguished by a
functional group that contains the element oxygen.
Carboxylic acids
• Members of the carboxylic acid family contain a carboxylic
acid group
• The carboxylic acid group comprises a hydroxyl group
connected to a carbonyl group:
O
C
carbonyl group
49
O
+
O
H
hydroxyl group
C
O
H
carboxylic acid group
Alcohols, Carboxylic Acids & Esters
Carboxylic acids
• The present of the hydroxyl group next to the cabonyl
group completely changes it properties.
•
The alcohol hydroxyl group and the carboxylic acid hydroxyl group are
chemically quite different, which is why molecules that have the carboxylic acid
group are placed in a separate family from the alcohols.
•
Later in the semester we will learn about some of these chemical differences.
O
C
carbonyl group
50
O
+
O
H
hydroxyl group
C
O
H
carboxylic acid group
Alcohols, Carboxylic Acids & Esters
Carboxylic acids
• The carboxylic acid group can be attached to a hydrogen,
an alkane-type carbon, or an aromatic-type carbon:
O
H
C
OH
methanoic acid
(formic acid)
51
H
H
H
O
C
C
C
H
H
O
OH
propanoic acid
C
OH
benzoic acid
Alcohols, Carboxylic Acids & Esters
We look now at three families that are distinguished by a
functional group that contains the element oxygen.
Esters
• Chemically, esters can be synthesized by reacting a
carboxylic acid with and alcohol:
O
C
O
O
H
carboxylic
acid
52
+
H
O
C
alcohol
C
O
C
ester
+
H
O
H
water
Alcohols, Carboxylic Acids & Esters
We look now at three families that are distinguished by a
functional group that contains the element oxygen.
Esters
• Chemically, esters can be synthesize by reacting a
carboxylic acid with and alcohol:
O
CH3
CH2
Carboxylic
acid part
C
O
CH2 CH3
Alcohol
part
Ethyl propanoate
53
Alcohols, Carboxylic Acids & Esters
Carboxylic acids
• The carboxylic acid group can be attached to a hydrogen,
an alkane-type carbon, or an aromatic-type carbon:
O
H
C
OH
methanoic acid
(formic acid)
54
H
H
H
O
C
C
C
H
H
O
OH
propanoic acid
C
OH
benzoic acid
Alcohols, Carboxylic Acids & Esters
As we saw with the hydrocarbons, the physical properties of
organic molecules depend on the noncovalent intermolecular
interactions which attract one one molecule to another.
•
With hydrocarbons, there is only one type of noncovalent
interaction:
•
•
The presence of the electronegative oxygen makes
alcohols, carboxylic acids and esters polar molecules,
these families, therefore, have at least two types of
noncovalent interactions:
•
•
55
Induced dipole/Induced dipole (London dispersion force)
Induced dipole/Induced dipole (London dispersion force)
Dipole/Dipole
Alcohols, Carboxylic Acids & Esters
As we saw with the hydrocarbons, the physical properties of
organic molecules depend on the noncovalent intermolecular
interactions which attract one one molecule to another.
•
Alcohols and Carboxylic acids also have a hydroxyl group
with a hydrogen bonded to an oxygen. This allows them to
form hydrogen bonds with each other. Therefore,
carboxylic acids have at least three different noncovalent
interactions:
•
•
•
56
Induced dipole/Induced dipole (London dispersion force)
Dipole/Dipole
Hydrogen bond
Alcohols, Carboxylic Acids & Esters
To summarize, the types of noncovalent interact ions that
each family can participate in include:
•
Hydrocarbons (Alkanes, Alkenes, Alkynes &
Aromatics)
•
•
•
57
Induced dipole/Induced dipole (London dispersion force)
Esters
•
•
Induced dipole/Induced dipole (London dispersion force)
Dipole/Dipole
Alcohols & Carboxylic acids
• Induced dipole/Induced dipole (London dispersion force)
• Dipole/Dipole
• Hydrogen bond
Alcohols, Carboxylic Acids & Esters
These interactions are illustrated in Figure 4.23 of your
textbook.
alcohols
esters
carboxylic acids
58
Alcohols, Carboxylic Acids & Esters
Boiling points are a good measure of the strength of the
noncovalent interactions between molecules.
59
•
The stronger the interactions, the higher the boiling point
will be.
•
Since all molecules have the London dispersion
interaction, the boiling points of molecules is expected to
increase with temperature.
•
The next slide shows a chart using the data found in Table
4.7 of Raymond, in which the boiling points for alcohols,
carboxylic acids and esters are plotted against molecular
weight.
60
•
As expected, the boiling points
for members of all three
families increases with
molecular weight due to the
London dispersion interactions.
•
For a given molecular weight,
the alcohols and carboxylic
acids have a higher boiling
point than esters, this is
because they can form
hydrogen bonds and esters
cannot.
•
The carboxylic acids have a
slightly higher boiling point
than alcohols, because they
can form two hydrogen bonds
with a neighboring molecule
(See Figure 4.23 in Raymond)
Boiling Point {°C}
Alcohols, Carboxylic Acids & Esters
Molecular Weight {g/mol}
Alcohols, Carboxylic Acids & Esters
Another distinguishing characteristic of many of the families is
odor.
•
•
You nose is actually a highly sensitive chemical detector.
•
For example:
The members of different families can interact differently
with the receptors in your nose to produce smells that are
characteristic of the families they belong to.
•
Carboxylic acids produce the pungent, sometime unpleasant odors associated
with ripe cheeses, rancid butter and vomit.
• Esters, on the other hand, produce the sweet, often pleasant order associated
with flowers, perfumes and various natural and artificial flavorings. The next
slide shows Figure 4.24 from Raymond, which gives some specific examples.
61
Alcohols, Carboxylic Acids & Esters
Examples of some
flavorable esters:
62
The End