* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dr. Schaaf - National Academies
Survey
Document related concepts
Polysubstance dependence wikipedia , lookup
Drug design wikipedia , lookup
Orphan drug wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug interaction wikipedia , lookup
Transcript
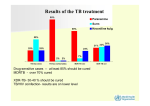
Drug-resistant tuberculosis in children HS Schaaf Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Stellenbosch University, and Tygerberg Children’s Hospital Introduction § WHO estimated that 511 000 new cases of MDR-TB occurred in 2007, 4.9% of all TB cases § South Africa estimated to contribute 16 000 new MDRTB cases per year – 4th amongst the 27 high-burden MDR-TB countries world-wide (7000 notified cases) § XDR-TB in every province – both in HIV-infected and uninfected patients. § Lately, RMP mono-resistance seems on the increase in especially HIV-infected adults MDR-TB mainly a man-made disease § Poor management of drug-susceptible or monoresistant TB cases, e.g. incorrect regimens (adding a single drug or weak combinations), interrupting treatment (drug supplies, poor bioavailibility), poor adherence by patients § Poor management of MDR-TB cases leads to transmission of MDR-TB strains, e.g. late diagnosis, not doing DST in relapse/retreatment cases, poor infection control measures especially in high prevalence HIV settings Lessons from case? § Adult TB cases: TAKE A HISTORY! – know when to screen for MDR-TB § Child contacts – screen for TB disease – if no disease, appropriate prophylaxis – regimen, timing & follow-up § Screen with CXR and interpret correctly § TB disease: Treat children according to DST of adult source case until result of own culture and DST available § Good weight gain not always a sign of good clinical response § What about management of MDR-TB in pregnancy? § MDR-TB is a curable disease (and pre-XDR TB) MDR-TB in children § Is mainly new (transmitted) drug resistance – this has been confirmed with DST and DNA-fingerprinting § Is more difficult to acquire because of the paucibacillary nature of primary disease, but is possible with cavitary pulmonary disease § In our experience DR-TB is not less infectious and does not cause less disease than DS-TB § Disease in children usually (>90%) develops within 12 months of infection § Contact tracing and follow-up of children exposed to MDR/XDR-TB should receive high priority Drug resistance surveys amongst childhood TB cases reported after 2000 Country Year Central African Rep INH-R MDR 1998-2000 25/165 15 (9.1%) 1 (0.6%) Egypt ? 4 (5.4%) 2 (2.7%) Greece 1994-2004 16/77 12 (15.6%) 3 (3.9%) NA (10%) NA(3.5%) 1 (1%) 0 India – TB Res Centre 1996 Madagascar Any DR 18/73 ?/201 1997-2000 1/97 Epidemiology: 4 surveys TCH in WCP Characteristics 1994-98 2003-05 2005-07 2007-09 All cult+ cases 338 (%) 323 (%) 291 (%) 294 (%) Med age (yrs) 2.6 2.5 2.75 2.1 193 (57.1) 173 (53.6) 154 (52.9) 156 (53.1) 32 (9.5) 59 (18.3) 65 (22.3) 50 (17.0) HIV test done 166 (49.1) 243 (75.2) 174 (59.8) 217 (73.8) HIV-infected* 13 (7.8) 64 (26.3) 49 (28.2) 63 (29.0) Boys Prev TB Rx *As percentage of tests done Significant increases in previously treated children and HIV-infected children from 1st to 2nd survey Epidemiology: 4 surveys in WCP of SA DST results 1994-98 2003-05 2005-07 2007-09 All cult+ cases 338 (%) 323 (%) 291 (%) 294 (%) 306 (90.5) 320 (99.1) 285 (97.9) 292 (99.3) Any DR 21 (6.9) 41 (12.8) 43 (15.1) INH mono-R 14 (4.6) 22 (6.9) 22 (7.7) RMP mono-R 0 0 2 (0.7) 7 (2.3) 19 (5.9) 19 (6.7) DSTs done* MDR *Further percentages based on number of DST results No significant differences in last 3 surveillance periods Ethambutol resistance DST results EMB-R in any DR tested EMB-R in MDR cases 2003-05 2005-07 2007-09 1/36 (2.8) 4/27 (14.8) 13/36 (36.1) 1/16 (6.3) 4/17 (23.5) 12/24 (50.0) § P = 0.001 and 0.01 for EMB resistance in any DR and MDR cases, respectively § None had confirmed XDR, although 1 child was a contact of an XDR-TB case § 2 had resistance to ofloxacin and 1 to amikacin New DR vs. Previously Rx DR (2003-2007) Results New cases Previous Rx OR (95%CI) Number with DST 481 (%) 123 (%) Any DR 47 (9.8) 36 (29.3) 0.26 (0.16-0.45) MDR 15 (3.1) 21 (17.1) 0.16 (0.08-0.34) 29 of 36 (81%) previously treated cases transmitted DR: § 5 MDR contacts failed H or HR prophylaxis § 6 more index cases (parents) MDR TB § 3 confirmed DR reinfection with RFLP § 2 original cultures MDR missed (wrong Rx) § 10 no response to adherent Rx (5 index cases died, no DST) § 3 INH-R cases completed adherent Rx >1yr before – 2 new contacts (1 died) and 1 with CD4<1% History of contact and DST comparisons § Concordance between DST in source cases to DST in child contacts was approximately 78% in these studies § Therefore - Need to try and confirm drug resistance in the child § While awaiting culture/DST results in the child, treat according to adult source case’s DST result Messages from surveilllance § These surveys show a high prevalence of DR and MDRTB in the WCP § Despite previous treatment, >90% of all DR-TB in children was most likely transmitted DR § Although hospital-based surveys are potentially biased, we did not find a significant difference in a communitybased vs. hospital-based survey (data not shown) § DR not significantly different between HIV-infected and HIV-uninfected cases § The few DSTs known in adult source cases hampers effective management of child TB contacts Diagnosis : M/XDR-TB in children § DR TB is a microbiological diagnosis § In children often difficult (paucibacillary TB): ▫ Confirmed if DR M. tuberculosis strain is isolated from a child ▫ Probable DR-TB if known contact with an adult DR PTB case ▫ Suspect DR-TB if: • a child gets worse on Rx, failing adherent Rx • an adult source case with unknown DST result is a treatment failure, a retreatment case or died of TB during adherent Rx Management of MDR-TB in children § Confirm MDR-TB if at all possible § If MDR TB is confirmed, also do DST for 2nd-line drugs § Management – at a specialized MDR-TB clinic § Use 1st-line drugs to which isolate is susceptible plus 2nd-line drugs (50% MDR children EMB-resistant!) § Be aware of different drug groups and cross-resistance § 2nd-line drugs are generally more toxic than 1st-line drugs § Adverse events more difficult to assess in children Principles of childhood MDR-TB Rx § NEVER add one drug to a failing regimen § DOT with daily treatment only is essential § Give 3-4 or more drugs to which the patient’s isolate is susceptible and/or naïve (extent of disease) § Use the adult index case’s isolate DST pattern if no isolate from child is available § Counsel patients/parents at every visit for support, about adverse events, and importance of adherence § Follow-up is essential; clinical, radiological and cultures 11-month-old Morning tablets MDR-TB Rx Difficult to accurately break tablets to correct dosage. Also receives an injectable Drugs for treatment of MDR-TB Group 1 -Remaining First-Line Drugs Drug Daily dosage mg/kg Maximum Ethambutol↔ PZA 20-25 25-40 2.5g 2.0g INH (Group 5) 15-20 400mg 1200 High-dose INH combined with ETH could give one effective drug Cannot rely on any of these drugs 2nd-line or reserve anti-TB drugs Group 2: Second line injectables Drug Aminoglycosides: - Kanamycin - Amikacin Capreomycin 120 0 Unit size Daily dosage mg/kg Maximum in mg (SA) 15-30 15-30 15-30 1.0 g 1.0 g 1.0 g 1000 100/250/500 1000 Drugs for treatment of MDR-TB Group 3: Fluoroquinolones Drug Fluoroquinolones: ↔ - Ofloxacin - Levofloxacin - Moxifloxacin 15-20 7.5-10 7.5-10 800mg 750mg 400mg 200/400 250/500 400 - Ciprofloxacin 30-40 2.0g 250/5ml 250/500 (Ciprofloxacin NOT recommended) 1200 Daily dosage Unit size mg/kg Maximum in mg (SA) 2nd-line or reserve anti-TB drugs Group 4: Second-line oral bacteriostatic drugs Drug Unit size Daily dosage mg/kg Maximum in mg (SA) Ethionamide/ Prothionamide 15-20 1.0g 250 Cycloserine/ Terizidone 10-20 750 mg 250 8-12.0g 4g sachets PAS 150 (2-3 (para-aminosalisylic divided acid) doses) 120 0 2nd-line or reserve anti-TB drugs Group 5: Drugs of unclear role in DR-TB treatment Drug Daily dosage mg/kg Maximum Linezolid (New) 10 mg/kg/dose 12 hrly 600/day? Clarithromycin 7.5 mg/kg/dose 12 hrly 1000/day Amoxicillin/ Clavulanic acid 10-15 (amox) mg/kg/dose 8 hrly Clofazimine Thioacetazone Imipenem/ cilastatin 1200 Do not use in HIV-infected patient Side effects 2nd-line drugs Drug Side effects Tests Amikacin/ ototoxicity, hearing test, Kanamycin/ nephrotoxicity creatinine, K+ levels Capreomycin Ethionamide Fl-quinolones GIT disturbance (split dose, escalate) hepatotoxicity, ALT? hypothyroidism TSH (T4) GIT disturbance± clinical observation insomnia, arthralgia Side effects 2nd-line drugs Drug Side effects Tests Cycloserine/ Terizidone psychosis, depression, parasthesias GIT disturbance hypothyroidism myelosuppression periph neuropathy lactic acidosis pancreatitis more common in adults PAS Linezolid add pyridoxine TSH (T4) FBC -Hb, platelets, WCC clinical lactate clinical, amilase? Duration of treatment § Optimal duration of treatment in children is not known § Cavitary or extensive pulmonary TB: as for adults 18 months after first negative culture § Primary, non-cavitary MDR-TB - Often culturenegative (paucibacillary): 12-15 months treatment probably sufficient. § Intensive phase including 2nd-line injectable drug, continuation phase mainly stop injectable drug Outcome § Few studies on outcome of DR-TB in children § Peru – cure/Rx completed obtained in 36 of 38 (95%) MDR-TB cases [Drobac et al Pediatrics 2006] § South Africa – study of 39 culture-confirmed childhood MDR-TB cases had 4 (10%) deaths and a majority of children clinically cured [Schaaf et al ADC 2003] § Need more morbidity and long-term mortality data – HIV-infected/uninfected Prophylaxis for DR-TB contacts § No RCT available. WHO 2006 MDR guidelines: INH only (possible DS contact) or clinical follow-up § Failure of INH or INH/RMP to prevent MDR TB reported. § INH mono-resistance: RMP x 4 mo § RMP-monoresistance: INH x 6 mo (Line-Probe test?) § MDR-TB: INH (15-20mg/kg) + EMB or ETH + OFL x6 mo § Pre-XDR or XDR-TB – only high-dose INH (1520mg/kg)? § In both MDR and XDR-TB regular follow-up for a minimum of 12 months (WHO recommends 2 yrs) § Acknowledgements - Peter Donald - Robert Gie - Nulda Beyers - Ben Marais - Anneke Hesseling - Rob Warren - Wendy Brittle - Tommy Victor - Paul van Helden - Nursing Staff and Research Assistants - The Children and their Parents § Financial Support - SA MRC - Harry & Doris Crossley Foundation - National Research Foundation SA