* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Synthesis II
Survey
Document related concepts
Enantioselective synthesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Bottromycin wikipedia , lookup
Ene reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Elias James Corey wikipedia , lookup
Aldol reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Kinetic resolution wikipedia , lookup
Asymmetric induction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Hydroformylation wikipedia , lookup
Transcript
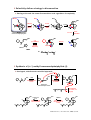
Organic Synthesis II: Selectivity & Control 8 lectures, TT 2011 MeO O H HN Handout 1 Handouts will be available at: http://msmith.chem.ox.ac.uk/teaching.html Dr Martin Smith Office: CRL 1st floor 30.087 Telephone: (2) 85103 Email: [email protected] ! Organic Synthesis II: Selectivity & Control. Handout 1 ! Selectivity and Control ! Definitions: Chemo- and Regio-selectivity ! Recap of selective reactions: reductive amination 1,4 vs 1,2 addition Electrophilic aromatic substitution Nucleophilic aromatic substitution ! Stereoselectivity: definitions and recap ! Selectivity and disconnection ! The finished product: total synthesis of (+-) methyl homosecodaphniphyllate ! Chemo- and Regio-selectivity in oxidation of alcohols ! Oxidation as a common functional group interconversion ! Oxidation of alcohols: Cr(VI) oxidants ! Activated DMSO oxidants: Swern, Moffatt and Parikh-Doering procedures ! Application to the generation of bis-aldehydes : (+-) methyl homosecodaphniphyllate ! Hypervalent iodine: Dess-Martin periodinane ! MnO2 and Oppenauer Oxidations ! Catalytic Oxidants: TPAP and TEMPO ! Chemo- and Regio-selectivity in reduction of carbonyl derivatives ! Selectivity in DIBALH reductions: stopping reactions half-way ! Selectivity in (general) hydride reductions ! Using amides as electrophiles: Weinreb amides and examples; the problem of C-acylation ! An aside: acylation at carbon - kinetic and thermodynamic control ! Kinetic control: use of methylcyanoformate ! Selectivity in hydride reducing agents: Lithium Aluminium Hydride Lithium Borohydride Borane (and related complexes) NaBH4; modified borohydrides & the Luche reduction ! Organic Synthesis II: Selectivity & Control. Handout 1 !Stereoselectivity in hydride reductions: 1,2 stereoinduction (Felkin models and variants) 1,3 stereoinduction (1,3-syn and 1,3-anti diols Additions to cyclohexanones (torsional control) Enantioselectivity in hydride reduction A catalytic asymmetric hydride reduction ! Recap: reduction of alkynes ! Dissolving metal reductions: the Birch reduction ! Dissolving metal reductions of !,"-unsaturated ketones (and esters) ! Hydrogenation ! Oxidation reactions involving alkenes ! Recap: dihydroxylation and allylic alcohol reactions ! Osmium-mediated hydroxylation ! Allylic alcohol mediated alkene functionalization ! Titanium mediated epoxidation: the Sharpless Epoxidation ! The Wacker oxidation ! Epoxidation vs Baeyer-Villiger ! Nucleophilic epoxidation of electron deficient alkenes ! Books & other resources: 1. Oxidation & Reduction in Organic Synthesis (T. J. Donohoe, OUP) 2. Organic Chemistry (Clayden, Greene, Wothers & Warren, OUP) 3. Professor Andrew Myers website (Harvard). http://www.chem.harvard.edu/groups/myers/page8/page8.html 4. Molecular Orbitals and Organic Chemical Reactions (I. Fleming, Wiley, 2nd Edn.) Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ! What is ‘Selectivity & Control’? (and why do we need it?) ! Chemo-selectivity Selectivity between two functional groups with nucleophiles or reducing agents O Which group reacts? O O OMe reaction with peroxy-acids ! Regio-selectivity Selectivity between different aspects of the same functional group direct or conjugate addition with nucleophiles or reducing agents Where does it react? X ortho-, para- or metawith electrophiles and selectivity between o- and p- O ! Chemoselectivity: reactions you have already seen… ! Functional groups have different kinds of reactivity NaBH4 OH H2 O O C=C weaker #bond than C=O Pd/C use nucleophilic reagent ketoneelectrophilic alkene- not electrophilic use catalytic hydrogenation ! Functional groups have similar reactivity OH O NaBH4 O OMe use selective reagent O O OMe ketone- more electrophilic ester- less electrophilic OH protect more reactive group ! Chemoselectivity: reactions you have already seen… ! Functional group may react twice (is the product more reactive than the SM?) control? R1 R2 Br NH2 R1 R2 N H Br R2 R1 R2 N R2 Chemoselectivity needed between Starting Material and Product ! Solution: use reductive amination (more on reduction later) H R1 O NH2 H R2 R1 R2 N NaB(CN)H3 R1 R2 N H or H2, Pd/C Chemoselectivity: NaBH3CN only reduces imine, not aldehyde starting material [NaBH3CN is a less nucleophilic source of hydride than NaBH4 due to the electron-withdrawing nature of the cyanide ligand. As a consequence it will generally not reduce aldehydes and ketones at neutral pH] ! Regioselectivity: reactions you have already seen… ! Conjugate and Direct Addition to Enones kinetic product OH O Direct Nu Nu thermodynamic product Conjugate [Michael] O Nu Nu hard nucleophiles the enone is electrophilic at two different sites soft nucleophiles ! Electrophilic Aromatic substitution X X X E E X E and/or E E choose 'ortho, para-'directing group X: Alk, OH, F etc choose 'meta'directing group X COR, NO2 etc ! What is ‘Selectivity & Control’ ? (and why do we need it?) ! Stereo-selectivity Selectivity between two (or more!) possible stereochemical outcomes Examples you’ve already seen: (I) reduction of cyclohexanones (see course from Dr E. Anderson, HT 2011) "H- " O Which face is attacked? H OH 'Hydride' tBu H tBu H H "H- " OH tBu H equatorial attack axial attack Examples you’ve already seen: (II) Additions to chiral aldehydes & ketones (Felkin-Anh model) O Ph Me Me OH LiBH(s-Bu)3 Ph [bulky hydride] Me Me 1. Reactive conformation 2. Bürgi-Dunitz trajectory 3. Attack away from RL and over RS 4. TS is SM-like RM O RL Nu RS H ! Selectivity defines strategy in disconnection ! Reminder of basic principles: where and when to disconnect? (i) branch points (ii) heteroatoms (iii) functional groups (iv) simplifying transformations (v) the order of events (see 1st year course from Prof Gouverneur) ! Disconnections that require selectivity: simple aromatic derivatives O O2 N O NO2 Esterification Dinocap - fungicide directs orthoand para- for nitration OH C-O OH O2 N Friedel Crafts (issues?) C-N Nitration O2 N NO2 C-C OH directs orthofor C-C bond formation NO2 para- position blocked Order is important (the alternative C-N disconnection prior to the C-C disconnection would not lead to appropriate selectivity) ! Selectivity defines strategy in disconnection ! The 1,2 difunctional disconnection OH R2N OH RS OH HO ! Two group disconnections: Fluconazole 2 N N OH N 1 F N C-N N 1,2 di-X 2 NH N N N sulfur ylid F N O 1 F N N F F Deactivating: only monoacylation F is o-, pdirecting F O Cl X- least hindered end attacked N N F + 1,2 di-X O OH 2 N OH X Cl F C-C Friedel Crafts C-N 1,2 di-X O Cl NH N F N F ! Complex molecule synthesis ! We need to exert control to be able to construct complex molecules efficiently ! Selectivity - as defined by disconnection - offers an opportunity to do this CO2Me H HN Methyl homosecodaphniphyllate ! Isolated from the bark of Daphiphyllum macropodum ! Structure confirmed by X-ray crystallography ! Complex architecture contains five fused rings ! Selectivity defines strategy in disconnection ! Starting at the end: the same disconnections work regardless of complexity BnO BnO MeO2C OBn H 1 H FGI (ox) 1,1 C-X (Prins) HN H redrawn Diels Alder N H H 2 x C-C H N N H R Methyl homosecodaphniphyllate imine formation 2 x C-N OBn OBn C-C (1,4 addition) N O N I OBn H O FGI (ox) O O MeO2C C-C (enolate alkyation) MeO2C H R R R R= ! Synthesis of (+/-) methyl homosecodaphniphyllate (I) ! An elegant, selective and controlled approach: MeO OBn H N 1. LDA O H N N Generate lithium enolate O OBn OBn Regioselective 1,4 addition OLi O LiO OMe Amide gives predominantly Z enolate SN2 alkylation [on carbon, not oxygen] I H O O OBn 1. KOH, 95˚C 2. H+ OBn N Amide hydrolysis and lactonization O HO H OBn DIBALH N Chemoselective reduction (ester vs amide) H O MeO2C Heathcock et al., J. Am. Chem. Soc., 1988, 10, 8734 ! Synthesis of (+/-) methyl homosecodaphniphyllate (II) ! An elegant, selective and controlled approach: OBn 1.LiAlH4 H O HO Chemoselective reduction of lactone to diol O OBn (COCl)2, DMSO Et3N, CH2Cl2 H OBn O Swern Oxidation Chemoselective oxidation to bis-aldehyde HO H O NH3 OBn OBn H H N N N-protonation OBn NH3 AcOH H Imine formation H O Enamine formation HN ! Synthesis of (+/-) methyl homosecodaphniphyllate (III) ! An elegant, selective and controlled approach: BnO OBn !2s H AcOH, 25˚C redrawn as: H H N H N Regioselective and Chemoselective intramolecular Diels Alder reaction H N !4s !4s + !2s BnO BnO BnO AcOH, 75˚C -H+ H H HN HN Prins reaction Electron rich alkene traps electron poor iminium cation (to give a tertiary carbocation) H H N ! Synthesis of (+/-) methyl homosecodaphniphyllate (IV) ! Final steps: BnO HO H2, Pd-C H H Removes benzyl protecting group and reduces alkene HN CO2Me 1. CrO3, H2SO4, H2O, Acetone 2. MeOH, H+ H Chemoselective oxidation to acid; Esterification HN HN ! A series of simple but selective steps ! Chemo-selectivity, regio-selectivity (and stereoselectivity) are exploited throughout the synthesis to great effect. ! Overall: nine steps - a spectacularly elegant and efficient approach We will cover the details of many of these steps throughout the course ! Oxidation is a very common synthetic transformation ! Many functional group transformations are redox reactions bromonium cation electrophilic attack elimination Br SN2 Br Br Br inversion Br Br Enters as Br+ Base -2HBr dibromide product Leaves as Br- 2 electron oxidation Oxidation = Electrophilic attack = Removal of electrons So functional groups that react readily with electrophiles are easily oxidized This includes: alcohols, alkenes, amines and phenols ! Common 2-electron transformations: -2e R OH alcohol -2e R O aldehyde -2e O R R OH acid NH2 amine -2e R imine NH NH R nitrile ! Selectivity in oxidation of alcohols ! Selective oxidation is a challenging transformation +H2O [ox] R R OH primary alcohol O [ox] OH -H2O R aldehyde OH O R hydrate OH acid Generally use Cr(VI), Mn(VII), high oxidation state Sulfur or Iodine Problems: aldehydes are reactive so over-oxidation can be a problem - avoid water! ! Reagents you’ve seen: Cr(VI) oxidation to generate ketones (idealized mech.) Cr(VI) O Cr Cr(IV) O H O OH OH O RDS Cr O O O Chromate Ester ! Selectivity: variants of chromium oxidants ! Common Cr reagents: H2Cr2O7 Jones reagent Chromic acid in H2SO4 Usually acetone co-solvent OTBS BnO O Oxidizes 1˚ alcohols to carboxylic acids; 2˚ alcohols to ketones. Not suitable for acid sensitive substrates Jones reagent BnO CO2H O N H Cr2O722 Pyridinium dichromate (PDC) PDC in dry DCM works well for 1˚ alcohol to aldehyde oxidation. In DMF oxidation to acids occurs CO2Me N H Acid-mediated desilylation and subsequent oxidation TBS =tbutyldimethylsilyl Bn = benzyl CO2Me ClCrO3- Pyridinium chlorochromate (PCC) PCC will oxidize 2˚ alcohols to ketones and 1˚ alcohols to aldehydes (if kept dry!) Selective oxidation can be problematic with these reagents & stoichiometric chromium can create waste disposal problems - so many alternatives. ! Selectivity in oxidation of alcohols ! The Swern oxidation: (i) activation of DMSO Chlorosulfonium salt O O Cl Cl S -78˚C O ClS DCM S Cl O O O + C + CO O Cl O Oxalyl chloride DMSO Chlorosulfonium salt is effectively the oxidant - unstable above approx -60˚C ! The Swern oxidation: (ii) activation of the alcohol S R1 OH Cl R1 primary alcohol H NEt3 H -HCl O S H R1 alkoxysulfonium salt H O + S R1 Me2S O aldehyde alkoxysulfonium ylide 1. Effective for primary and secondary alcohols 2. Amine base is necessary to facilitate breakdown of alkoxysulfonium salt This is an excellent method for the selective oxidation of primary alcohols to aldehydes ! Selectivity in oxidation of alcohols ! The Swern oxidation: (i) activation of DMSO Chlorosulfonium salt O O Cl Cl S -78˚C O ClS DCM O O S Cl O + C + CO O Cl O Oxalyl chloride DMSO Chlorosulfonium salt is effectively the oxidant - unstable above approx -60˚C ! The Swern oxidation: (ii) activation of the alcohol S R1 OH primary alcohol Cl R1 H NEt3 H -HCl O S H alkoxysulfonium salt R1 H O + S alkoxysulfonium ylide R1 O aldehyde 1. Effective for primary and secondary alcohols 2. Amine base is necessary to facilitate breakdown of alkoxysulfonium salt This is an excellent method for the selective oxidation of primary alcohols to aldehydes Me2S ! Swern oxidation: methyl homosecodaphniphyllate ! Revisit complex example: the order of addition is important Key: never unmask aldehyde in the presence of reactive functional groups OBn HO HO then add Et3N H O -78˚C OBn OBn S (COCl)2, DMSO, CH2Cl2 H O O O S H bis-alkoxysulfonium salt mono-oxidation [generates mono-aldehyde] in situ OBn H O OBn hemiacetal formation H HO HO OBn further oxidation H O O O There is no control over which alcohol is oxidised first in the ‘lower’ route. ! Alcohol oxidation: other DMSO-based reagents ! Parikh-Doering oxidation (SO3.pyridine is the DMSO activator) OTBS OTBS SO3.py, Et3N S H S HO S CH2Cl2/DMSO, rt SO3.pyr is a crystalline solid Mild and scaleable oxidation Selective for 1˚ alcohol ^ aldehyde H S HO O HO No diol cleavage ! Pfitzer-Moffat oxidation (DCC is the DMSO activator) N OtBu Cl OH DMSO, DCC Cl H+, pyridine Possible side reactions: Pummerer rearrangement, and acid-induced problems for sensitive substrates C N OtBu O DCC = Dicylclohexylcarbodiimide [the dicylohexylurea biproduct of this reaction can be difficult to remove from the reaction mixture] Mechanisms are essentially the same as the Swern, but with the electrophilic DMSO activator being SO3 or DCC ! Dess-Martin Periodinane (‘DMP’) ! Hypervalent iodine oxidant: mild, reliable and works at RT (stylized mech.) AcO I OAc OAc O R2 HO 2 R1 R H O AcO I O O 1.5 eq DMP DCM, RT R1 O OAc O I O O O R1 R2 O AcOH biproduct can be buffered for very sensitve substrates ! Examples (often used for complex, highly functionalized materials) Me Me Me Me H Me H TBSO 1.5 eq DMP Me H Me DCM, RT TBSO O H RT MeO I O DMP O O I O O Me MeO O OH O HO Selective for oxidation of 1˚ alcohols to aldehydes with no overoxidation ! Dess-Martin Periodinane (‘DMP’) ! Mechanistically, the first step is ligand exchange so we would expect to be able to kinetically discriminate between primary and secondary alcohols: HO HO O HO OTBS O TBSO DMP O O O DCM, RT OTBS OMe OTBS TBSO OTBS OTBS OMe OTBS 1˚ alcohol oxidized preferentially; 2˚ alcohol left untouched ! Allylic and benzylic alcohols oxidized faster (but can & will oxidize 2˚ alcohols) O O O O DMP DCM, RT H HO 2˚ allylic alcohol oxidized faster than ‘normal’ 2˚ alcohol H HO OH O Limitations: can cleave diols (like periodate, another hypervalent iodine oxidant) ! MnO2: a selective oxidant for allylic and benzylic alcohols ! Selective for allylic and benzylic alcohols (will not usually oxidize 2˚ alcohols) OH OH MnO2 OH O DCM Allylic alcohol oxidized selectively ! Selectivity is probably a consequence of a radical mechanism Mn(IV) O OH Mn(IV) Mn HO O O R1 Mn R1 allylic/benzylic alcohol OH Mn(III) O O Mn Mn(II) OH O R1 H OH Mn R1 OH Manganate ester aldehyde/ketone Hydrogen abstraction is faster for allylic/benzylic alcohols (the radical that is produced is delocalized and hence more stable) ! MnO2: a selective oxidant for allylic and benzylic alcohols ! Selective for allylic and benzylic alcohols (will not usually oxidize 2˚ alcohols) MnO2 Bu3Sn OH CH2Cl2 O Bu3Sn Mild conditions; can retain vinyl stannane group ! The aldehyde products can be used in-situ with other reagents (MnO2 is v. mild) Oxidant (MnO2) compatible with reductant (NaBH4) in same vessel OH MnO2, CH2Cl2 NaBH4, 4Å sieves amine N [red] N then methanol aldehyde formed in-situ, condenses with amine to form intermediate imine NaBH4 reduction of imine faster in polar protic solvent (MeOH) MnO2 is a heterogeneous oxidant: workup is generally just filtration ! A Catalytic Oxidant: Tetra-N-Propyl Ammonium Perruthenate ‘TPAP’ ! Ru(VII) with organic-soluble counterion is a selective oxidant and can be used catalytically with a co-oxidant (mechanistically complex!) O Pr O O Pr O O Pr N Ru Pr R2 HO Ru(VII) N R1 O O +2e Ru O 1˚/2˚ alcohol Ru O Ru(VI): active O Ru(IV): inactive O R2 O N O disproportionation Co-oxidant ‘NMO’ R1 O Aldehyde/ketone Ru O O Ru(V) Selective for oxidation of 1˚ alcohols to aldehydes with no overoxidation ! A Catalytic Oxidant: Tetra-N-Propyl Ammonium Perruthenate ‘TPAP’ ! Mechanism: consistent with ruthenate ester (though complex & unproven!) Ru(VII) O O Ru O O R2 R1 Ru(VII) H R2 R1 OH O O Ru O Ru(V) R1 OH Ruthenate ester 1˚/2˚ alcohol OH R2 O Ru O O O aldehyde/ketone ! Examples (4A sieves remove water to prevent hydration and overoxidation) H MeO MeO MeO H O O TPAP (10 mol%) H O HO TBSO O H MeO MeO OTBS NMO, DCM 4Å sieves MeO H O O OTBS H O O TBSO O Limitations: Can cleave diols (like high valent Manganese and periodate) ! A Catalytic Oxidant: Tetra-N-Propyl Ammonium Perruthenate ‘TPAP’ ! Can use in conjunction with the Wittig reaction for reactive aldehydes O O O TPAP (10%), NMO DCM, 4Å sieves OH CO2Et O CO2Et Ph3P Stabilized ylide; E/Z >20:1 ! Steric selectivity can be exploited: generation of lactones (compare Swern) OH O TPAP (10%) NMO OH O O OH O O [ox] OH DCM 4Å sieves O O 1˚ alcohol O Lactol readily oxidized Lactone TPAP will also oxidize sulfur but not other heteroatoms ! Thermodynamics in Oxidation: the Oppenauer reaction ! The reverse of the Meerwin-Pondorrf-Verley reduction (see Dr E. Anderson course, HT 2011) O R1 OH R2 R3 H Metal Alkoxide R4 Oxidant R1 L R3 2 M R O L H O R4 OH R1 H O R2 Reduced R3 R4 Oxidized M = Al, Zr, B, Ru, ! Very mild, uses non-toxic reagents and can be employed on a large scale MeO MeO O O O NMe HO Cl3C O Al(OiPr) 3 NMe OH H Zr(OtBu)4 O Generally superceded by other oxidants, but inexpensive and non-toxic. O ! Oxoammonium-mediated oxidations ! Most common reagent is TEMPO: 2,2,6,6-tetramethyl-1-piperidinyloxy N Oxoammonium Actual oxidizing agent O TEMPO N I Cl2 R2 HO O or R1 1˚/2˚ alcohol disproportionation N AcO NaOCl I O OAc TEMPO R2 O or R1 Aldehyde/ketone 'BAIB' N OH Co-oxidant TEMPO is used catalytically with a co-oxidant ! Oxoammonium-mediated oxidations ! Mechanism: reoxidation R2 O Actual oxidant ‘oxoammonium’ N N O O R2 HO 1˚/2˚ alcohol H R2 R1 N R1 O OH aldehyde or ketone R1 ! Examples: PMBO N HO OH TEMPO NaOCl TBSO PMBO O OMe O TEMPO BAIB KHCO3 PMB = O N MeO TBSO Generally: mild, functional group tolerant reagent O OMe ! Selectivity in reduction: hydride reducing agents ! Functional groups have different kinds of reactivity NaBH4 OH H2 O O Pd/C use nucleophilic reagent ketoneelectrophilic use catalytic hydrogenation alkene- not electrophilic ! For chemoselective and regioselective hydride reduction - which reagent? O O 1. NaOH 2. BH3 HO O O NaBH4 CeCl3 EtO OH EtO LiAlH4 OH HO Important to use the correct hydride for the correct transformation! ! Selectivity: hydride reducing agents (not ALWAYS reliable..) Reduced " Not usually reduced ! Slow NHR R' H O O ! R imine R H aldehyde ! R R' R ketone R OR' ester ! ! NaBH4 ! LiBH4 H via Acid Chloride O O O H LiAlH4 (low temp) DIBALH (low temp) O R H O R O NR'2 R OH amide acid " " " ! ! " " ! ! ! " " LiAlH4 ! ! ! ! ! BH3 ! ! ! ! ! NaBH3CN OH R' NHR amine R OH 1˚ alcohol R R' 2˚ alcohol R OH 1˚ alcohol R NR'2 amine R OH 1˚ alcohol ! Selectivity in reduction: hydride reducing agents ! DIBALH (Di-Iso-Butyl Aluminium Hydride) Exists as H-bridged dimer but reacts as monomer. H Al H Al Al Al has empty p-orbital in monomer so is electrophilic. H ! DIBALH becomes a good reducing agent after reacting with a nucleophile R2Al H O R R OR destroys any excess DIBALH tetrahedral intermediate R R Al H O R2AlO H R OR DIBALH reduces most nucleophilic C=O group H , H2O H , H2O HO H R OR OR R unstable hemiacetal stable at low temperatures O H ester reduced to aldehyde with DIBALH at low temperature ! Selectivity in reduction: hydride reducing agents ! DIBALH is a powerful and less selective reagent at higher temperatures R2Al H O R tetrahedral intermediate R R Al H O R OR R2AlO R OR aldehyde - much more reactive than ester SM O H OR R OH H R H rapid reduction to alcohol at RT this intermediate is NOT stable ! Often used at RT for reduction of esters to alcohols O tBuO OTBS Ph MeO N H Complete reduction of ester to 1˚ alcohol with excess DIBALH at RT CH2Cl2, RT O tBuO DIBALH CH2Cl2, RT OTBS MeO Ph N H O O O DIBALH BF3.OEt2 Selective 1,2reduction OMe OEt OH O OH ! Stopping reactions half way: reduction ! Application: the reduction of lactones O O DIBALH O O H , H2O OAlR2 H Ph3P R HO OH H 2 equivalents R stable cyclic hemiacetal The reactive aldehyde is never unmasked in the presence of reactive functionality the tetrahedral intermediate is stable under the reaction conditions ! Is this approach a solution to a common problem? O R1 O R2MgBr or R2Li OR ester less electrophilic O OH R2MgBr R1 R1 or R2Li R2 ketone more electrophilic O BuLi R2 R2 product formed OH BuLi OMe note regioselectivity too: direct rather than conjugate addition ! Stopping reactions half way: Weinreb amides ! Solution: use amides as electrophiles Li O R OLi R H NMe2 H DMF NMe2 H2O O R O H H H NMe2 H As seen in ortho-lithiation R H stable under reaction conditions ! A Weinreb amide is a better solution to this problem Li O R1 R2 O Cl HN OMe R1 Me R2 R1 O Li OMe N Me N OMe Me a Weinreb amide H H2O R2 O Li OMe R1 N Me N R-Li R-MgX tetrahedral intermediate stabilised by coordination Li OLi R2 OH R1 Acceptable nucleophiles: LiN O OMe R1 RO R DIBALH LiAlH4 R2 Me decomposes during acidic work-up ! Examples of the use of Weinreb amides ! Grignard addition OTBS O Me OTBS N Ar O Me TBSO N O OMe OMe N OMe Me Me O O2 N Me O Ar tetrahedral intermediate stabilized by coordination ! DIBALH reduction OMe OMe OTBS N O O Mg N Ar Me THF, 0˚C 99% yield N O Me MeMgBr OMe Me Me OMe DIBALH Me Me TBSO OMe Me Me O O2 N H Me THF, -78˚C 95% yield OMe Me OMe ! Enolate additions Li O OLi O N Me O O tBu NMe CO2tBu O O 83 % yield OtBu THF, -78˚C OMe "-keto ester: C-acylation requires control tetrahedral intermediate stabilized by coordination ! Stopping reactions half way: Acylation at Carbon ! The problem: acylation of ketones can be difficult to control O O R1 O R2 OR ester O O R1 R2 R2 further reaction? ketone less electrophilic more electrophilic ! Solution: Employ kinetic or thermodynamic control in Claisen condensation O R1 OR2 R1 O O O R2O OR2 R1 OR2 R1 acylation at carbon H O R2O OR2 R1 electrophilic ketone O O R1 OR2 R1 stable, non-electrophilic enolate - the product under the reaction conditions Note: The final enolization is reversible, but the equilibrium lies over to the RHS ! Thermodynamic control ! Intramolecular (Dieckmann) condensation can offer a solution to C-acylation O O EtO OEt H O CO2Et O EtO CO2Et MeI CO2Et Me CO2Et Irreversible alkylation ! Regioselectivity through thermodynamic control O O O O EtO EtO2C OEt O EtO EtO OEt H CO2Et EtO Cannot form a stable enolate Can form a stable enolate Note: The final enolization is reversible, but the equilibrium lies over to the RHS ! Acylation at Carbon - Kinetic vs thermodynamic ! Enolate stability can control regiochemistry of C-acylation O CO2Me O NC O O OMe MeO LDA, -78˚C O OMe NaH, 0˚C Kinetic product CO2Me Thermodynamic product ! With reversible enolization conditions we get equilibration between all species CO2Me H CO2Me O O O O O O CO2Me CO2Me This enolate destabilized by interaction with aromatic C-H bond - precludes planarity No such destabilizing interaction - more stable enolate Note: The final enolization is reversible, but the equilibrium lies over to the RHS ! Acylation at Carbon - Kinetic vs thermodynamic ! Enolate stability can control regiochemistry of C-acylation O CO2Me O NC O O OMe MeO LDA, -78˚C O OMe NaH, 0˚C Kinetic product CO2Me Thermodynamic product ! Stabilizing the intermediate precludes proton transfer under reaction conditions O O O R1 LDA OLi R1 R2 NC Li O O slow R2 -78˚C fast R2 O OMe R1 R2 OMe CN OMe R1 ! Example: O O Me NC Me O OMe O Me LDA, -78˚C OMe Notes: pKa MeOH = 16, pKa HCN = 9.5 ! Specific reducing agents: Lithium Aluminium Hydride LiAlH4 ! Powerful and (somewhat) non-selective reducing agent: will reduce aldehydes, ketones, esters and amides (anomalous: see mechanism below) [AlH4]- or [AlH(4-n)ORn] - Li counterion important (reaction ineffective without it) H O R1 O N R2 R1 H 3 H R H Al H Li N O R2 R1 H R3 All 4 hydrides active in principle H H Al N H R2 H H Al H Retain C-N bond (compare with ester reduction) H H R1 R3 N H R2 R1 R3 Al is Lewis acidic H N R2 R3 Iminium much more reactive ! Examples: O H H N Me N Me MeO H O H O THF O O LiAlH4 O MeO H O H Reduces amide and leads to 1,2-reduction of !,"-unsaturated ketone (hard nucleophile) OH Me LiAlH4 THF HO HO Me Me Me Lactone (ester) reduction leads to diol production (note cleavage of C-O bond) O ! Specific reducing agents: Lithium Borohydride LiBH4 ! Typically used for the selective reduction of esters in the presence of amides, nitriles and carboxylic acids (will also reduce aldehydes and ketones) F F O2N O H N CO2Me OTBS N H O O2 N LiBH4 H N OH O OTBS N H THF O Ester is reduced (with cleavage of the C-O alkyl bond) but amide is left untouched ! Examples: HO CH3 MeO2C LiBH4 CO2H O HO CH3 O N THF HOH2C CO2H CO2Me LiBH4 N OH THF CN Ester is reduced (with cleavage of the C-O alkyl bond) but acid is left untouched CN Selective ester reduction; nitrile and amide are left untouched Can be made in situ: LiI or LiBr + NaBH4 ! Specific reducing agents: Sodium Borohydride NaBH4 ! Less reactive (than LiAlH4 & LiBH4), more selective. Generally used in MeOH or EtOH & will not usually reduce esters, epoxides, lactones, nitriles. Mech: Na+ counterion less important than solvent H OMe O R1 H O R2 R1 H H B H H H H R2 OR B H H NaBH4 slowly reacts with protic solvents (or alcohol products) to generate alkoxy borohydrides All 4 hydrides active in principle ! Examples: O I OH O O O I OMe O NaBH4 MeOH O NEt2 O O O 1. NaBH4, MeOH OMe 2. 6M HCl H ketone is reduced but ester and vinyl iodide are left untouched aldehyde is reduced but amide left untouched (by reduction, anyway…) O O ! The Luche reduction: NaBH4 + CeCl3.7H2O ! NaBH4 is not selective for 1,2 vs 1,4 reduction: CeCl3 increases selectivity O OH 1,2-reduction favored by addition of Ce salt OH CeCl3 accelerates rate of reaction of protic solvent with NaBH4 to generate alkoxyborohydrides Reductant MeOH NaBH4 51% 49% NaBH4, CeCl3 99% trace NaBH[4-n]OMen These are harder reducing agents and favour attack at the hard rather than the soft center ! Examples: O N3 CO2Me OH O H NaBH4 CeCl3.7H2O H MeOH O O NaBH4 CeCl3.7H2O MeOH OH O O OBn OBn N3 CO2Me Exclusive 1,2-reduction No ester reduction Exclusive 1,2-reduction ! Other modified Borohydrides ! NaBH3CN and NaBH(OAc)3: reagents of choice for reductive amination OTBS Ph O N H Me H Ph NaBH3CN CH2O O pH 5 N Me AcO OTBS Me O R H NaBH(OAc)3 SnCl2 N O H H Reduces intermediate imine/iminium NOT aldehyde: selective reductive amination Me N AcO H O R Me Reduces intermediate imine/iminium NOT aldehyde. SnCl2 Lewis acid accelerates iminium formation ! Super-HydrideTM: alkyl groups make it the most nucleophilic hydride source challenging SN2 (neopentyl) Ph Ph MsCl, Et3N HO MsO Ph LiEt3BH THF H The most nucleophilic hydride: especially effective for SN2 type reactions on activated leaving groups Electron donating groups increase hydride donor ability of B-H bond ! Specific reducing agents: Borane BH3 ! Reagent for rapid reduction of acids (compare LiAlH4); idealized mechanism: H O R1 B H O H THF H OH R1 O H+ workup R1 H H O R1 B O O R1 H H OR2 B H O ‘OR2’ group could be THF or substrate HO OH R1 H H B is Lewis acidic Only 1 eq. BH3 per acid required B OR2 H O -H2 H B OR2 B is Lewis acidic OR HO B H H O R1 H O R1 H H OR2 B H O ! Examples O O O H O BH3.THF Me Br H Me CO2Et OH Br CO2H BH3.THF HO2C Selective acid reduction - no reduction of lactone HO CO2Et Selective acid reduction No 1,4-reduction; no ester reduction ! Chemoselectivity with hydrides: recap NHR R' O H imine R H aldehyde ! R O O O ketone R' R OR' ester ! ! NaBH4 ! LiBH4 R O NR'2 R OH amide acid " " " ! ! " " ! ! ! " " LiAlH4 ! ! ! ! ! BH3 ! ! ! ! ! NaBH3CN OH R' NHR amine R OH 1˚ alcohol R R' 2˚ alcohol R OH 1˚ alcohol R NR'2 amine What about stereoselectivity in reduction? R OH 1˚ alcohol ! Diastereoselectivity with hydrides: 1,2-stereoinduction ! Felkin-Anh model (see Dr E. Anderson course HT 2011) O OH LiBH(s-Bu)3 Ph Me Ph Me THF Me Me O Me 1. Reactive conformation 2. Bürgi-Dunitz trajectory 3. Attack away from RL 4. TS is SM-like R R Ph B R H Me H ‘Felkin’ product ! Felkin Polar model O LiBH(s-Bu)3 Ph 1. Electronegative group is treated as large 2. #* C=O overlap with $* C-S (lower LUMO) 3. Forming C-Nu bond stabilized by C-X $* OH Ph Me THF Me SMe O iPr MeS R R B Me H H R ‘anti-Felkin’ product ! Felkin Chelation model Me Zn(BH4)2 Ph Me THF OMe Zn2+ 1. Lewis acid metal 2. electronegative group coordinates to metal 3. Controls reactive conformation OH O Ph OMe MeO O R Me R B R ‘Felkin’ product H Ph H ! Diastereoselectivity with hydrides: 1,3-stereoinduction ! 1,3 polyols are components of many natural products (+)-Roxaticin OH OH OH OH OH Me OH O OH OH OH HO O 1,3-syn OH 1,3-anti Me ! A disconnection approach indicates how to assemble the 1,3-arrangement OH OH R1 R2 OH FGI [red] R1 O OH FGI R2 [red] 1,3-syn OH R2 R2 1,3-anti Can we use the stereochemistry of this product to direct hydride reduction to afford either the syn- or anti- product? ! Diastereoselectivity with hydrides: 1,3-stereoinduction ! 1,3-syn diols may be generated by using a Lewis acid to favor intermolecular hydride delivery from the least hindered face: HOH R3B, MeOH O R1 R R1 NaBH4 R2 R2 Boron is Lewis acidic B O O OH OH R1 R Chair-like TS axial attack of hydride R2 1,3-syn ! 1,3-anti diols may be generated by using intramolecular delivery of the hydride nucleophile OH R1 H Me4NBH(OAc)3 O R2 H Boron is Lewis acidic H R1 H OAc O B O R2 H OH OAc OH R1 R2 1,3-anti Chair-like TS put substituents pseudo-equatorial Intramolecular delivery ! Diastereoselectivity with hydrides ! Size matters: addition to cyclohexanones (see Dr E. Anderson course HT 2011) HH H H OH H Small Hydride O H 'Axial attack' Large Hydride H OH H H 'Equatorial attack' LiAlH4 Small H9:1, axial/equat. attack H- Na(s-Bu)3BH Big H96:4, equat./axial attack So equatorial attack appears to be favoured, as it does not require the hydride to approach across the ring (where 1,3-diaxial interactions hinder trajectory) ! Why is axial attack then favoured for small hydrides (nucleophiles)? axial H O H equatorial Equatorial: O moves towards C-H, leading to higher torsional strain in the TS Axial: O moves away from C-H, leading to lower torsional strain in the TS ! Enantioselectivity with hydrides I ! How does Nature perform reductions? O S-(+)-lactate CO2 NH2 N Histidine H O O H O Enzyme holds groups in H appropriate position for reactivity (to stabilize TS) and provide selectivity NH2 N NAD R* N NH O lactate dehydrogenase NADH Asparagine N H CO2 H O H HN H OH R* H N H O H NH2 N NADH R* ! We can mimic this in the lab by using Mg2+ to control conformation Me lactatelike H O N O O Ph N O Ph Ph Me H Mg2+ O MeO- Ph O Mg2+ N Ph NADH-like O Me O O H OH OH (S)-(+)-enantiomer Ph Mg activates and controls conformation Ph MeO Aromatic biproduct (a pyridinium salt) Single enantiomer produced ! Enantioselectivity with hydrides II ! We can put the chiral group on the reagent too (‘chiral NaBH4’) H N Ph H Ph Made from Proline ! Ph N H3B B O R ‘CBS’ reagent Chiral reducing agent Ph H3B B O R O Ph Ph O 10 mol% cat. Me Trivalent boron is lewis acidic Hydride delivered from 'top' face Overall: boat-like arrangement BH3 used to regenerate active material BH3 Ph N H B H Me O H Ph OH Ph Me (R)-alcohol, 97% ee A highly effective reagent for enantioselective reduction of ketones H H Ph O Cl Ph B O R (1 mol%) BH3, THF Ph N N Ph R B OH Ph Cl 98:2 ratio of enantiomers Ph B O Bu (1 mol%) O TBSO Me Me BH3, THF OH TBSO Me Me 97:3 ratio of diastereoisomers ! Reduction of alkynes (recap of 1st year material) ! Overall cis- addition of hydrogen across the alkyne: ‘hydrogenation’ H2 (g) R1 R2 Lindlar catalyst H H R2 R1 H H hydrogen on catalyst surface cis alkene ! Overall trans- addition of hydrogen across the alkyne: ‘dissolving metal’ LUMO !* C C R2 H R1 Na N H NH3 (l) H R2 R1 NH3 (l) Anion adopts transconfiguration H R2 Na R1 NH3 (l) H R2 NH3 (l) R1 H R2 R1 H Isolated alkenes are not usually reduced under these conditions ! Dissolving metal reductions: The Birch reduction ! The Birch reduction can be used to partially reduce aromatic rings H H A range of metals can be used: Li, Na, K (sometimes even Ca and Mg) Na, NH3 (l), EtOH Kinetic product is nonconjugated diene H H ! The regiochemistry of the reduction depends on substitution OMe CO2H OMe H H Na, NH3 (l), EtOH H CO2H Na, NH3 (l), EtOH H H Electron-donating groups (OR, NR2, alkyl) give rise to this orientation of reduction H H Electron-withdrawing groups (CO2H, CO2 R, COR, CONR2, CN, Ar) give rise to this orientation of reduction ! Dissolving metal reductions: The Birch reduction ! Mechanism: reduction of arenes bearing EDG (OR, NR2, alkyl) NH3(l) Li (or Na) Li+ A dark blue solution of solvated electrons. This is the actual reducing agent in the Birch reduction e [NH3]n Addition of electron into benzene LUMO OMe Undergoes orthoprotonation (probably) OMe OMe H Na, NH3(l) EtOH "e " H H OEt RDS H OMe OMe H H Na, NH3(l) EtOH "e " H Delocalized radical anion H H H OEt OMe H 1. Reduction of alkyl benzenes and aryl ethers requires a proton source stronger than ammonia (usually an alcohol). 2. First protonation occurs ortho- as this is the site of highest charge (and gives the most stable intermediate) 3. Second protonation occurs para- to give the non conjugated product: kinetic control and a consequence of the pentadienyl anion HOMO having the largest coefficient in this position H Undergoes paraprotonation H H Isolated C=C are not usually reduced ! Dissolving metal reductions: The Birch reduction ! Mechanism: reduction of arenes bearing EWG (aryl, carbonyl, acid, nitrile) CO2H CO2 CO2 Na, NH3(l) CO2 Na, NH3(l) H OEt EtOH "e " H H H Addition of electron into benzene LUMO CO2 EtOH "e " H H Undergoes paraprotonation 1. Carboxylate will be deprotonated under reaction conditions 2. First protonation in para- position: site of highest charge (and most stable intermediate) 3. Kinetic and irreversible protonation to give non-conjugated product. H OEt H CO2H Undergoes ipsoprotonation H H Isolated C=C are not usually reduced ! Examples: Me OMe Na, NH (l) Me 3 OMe Me CO2H Na, NH (l) Me 3 EtOH EtOH Both EDG: direct reduction to same orientation One EWG and one EDG: both direct to same orientation CO2H ! Dissolving metal reductions: The Birch reduction ! The proton source is important OMe OMe OMe H Na, NH3(l) Na, NH3(l) H H EtOH H ‘more’ reduction without EtOH non-conjugated diene (with EtOH) ! NH3 functions as a proton source (to make NH2-) if there is nothing better ‘normal’ nonconjugated product OMe OMe OMe OMe H Na, NH3(l) thermodynamic conjugated product H NH2 H H OMe NH2- H H H H NH2- strong base can isomerize NH3 functions as proton donor conjugated product: further reduction Some reductions do not proceed without the proton source. Applications of the Birch reduction Reductions of conjugated alkenes O O H3C O O H3C H K, NH3 THF, -70˚C H H H Conjugated alkene is reduced more rapidly than electron-rich arene MeO MeO Reductive alkylation: alkylation of the pentadienyl anion intermediate OMe CO2tBu 1. K, NH3 1eq. tBuOH OMe CO2tBu iPr 2. iPrI CN OMe CN 1. Li, NH3 1eq. tBuOH One equivalent of BuOH to permit first para- protonation, then kinetic alkylation of pentadienyl anion OMe 2. Br t Cl Cl Regiochemistry of reduction a consequence of substitution; note alkylation via displacement of best leaving group