* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download aldehydes and ketones
Survey
Document related concepts
Enantioselective synthesis wikipedia , lookup
Marcus theory wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Discodermolide wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Elias James Corey wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Ene reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Transcript
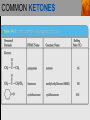
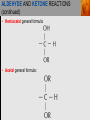
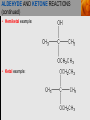
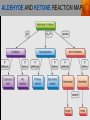
Spencer L. Seager Michael R. Slabaugh www.cengage.com/chemistry/seager Chapter 14 Aldehydes and Ketones Jennifer P. Harris ALDEHYDES AND KETONES • The carbonyl group: • Aldehydes have at least one hydrogen attached to the carbonyl group. • Ketones have two carbons attached to the carbonyl group. ALDEHYDES AND KETONES (continued) • Examples: NAMING ALDEHYDES • Find the longest carbon chain that contains the aldehyde group. • Change ending of the root hydrocarbon name by dropping –e and adding –al. • All other branches and groups are named and located using standard IUPAC system. • Examples: 3-bromobutanal 2-ethylbutanal COMMON ALDEHYDES NAMING KETONES • Find the longest chain that contains C=O. • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. • Examples: 3-methyl-2-pentanone 2-methylcyclohexanone COMMON KETONES PHYSICAL PROPERTIES • The carbonyl group is moderately polar, but it doesn’t have any hydrogen atoms bonded to the oxygen atom, so it cannot hydrogen bond between molecules. PHYSICAL PROPERTIES (continued) • Because of the polarity of the C=O group, these groups can interact, but the attraction is not as strong as hydrogen bonding. • This makes the boiling point of aldehydes and ketones higher than alkanes, but lower than alcohols. PHYSICAL PROPERTIES (continued) PHYSICAL PROPERTIES (continued) • The C=O group can hydrogen bond with water molecules because the oxygen atom in the carbonyl group has a partial negative charge that attracts the partial positive charge of a hydrogen atom in the water molecule. • This makes low molecular weight aldehydes and ketones water soluble (they have small hydrophobic sections). PHYSICAL PROPERTIES (continued) ALDEHYDE AND KETONE REACTIONS • Recall the oxidation of alcohols to produce aldehydes and ketones: ALDEHYDE AND KETONE REACTIONS (continued) • The difference in reactivity toward oxidation is the chief reason why aldehydes and ketones are classified in separated families. ALDEHYDE AND KETONE REACTIONS (continued) • The ease with which aldehydes are oxidized allows us to test for the presence of aldehydes with Tollens’ reagent or Benedict’s reagent. • In general, ketones fail to react with these reagents. From left to right, three test tubes containing potassium dichromate (K2Cr2O7), acetone, and benzaldehyde. After the addition of equal amounts of K2Cr2O7, the acetone remains unreacted, whereas the benzaldehyde is oxidized. ALDEHYDE AND KETONE REACTIONS (continued) • In the presence of aldehydes, Tollens’ reagent produces a silver coating on glass. ALDEHYDE AND KETONE REACTIONS (continued) • In the presence of aldehydes, Benedict’s reagent produces a red precipitate. From left to right, three test tubes containing Benedict’s reagent, 0.5% glucose solution, and 2.0% glucose solution. The addition of Benedict’s reagent from the first tube produces colors (due to the red Cu2O) that indicate the amount of glucose present. ALDEHYDE AND KETONE REACTIONS (continued) • The addition of H2 in the presence of catalysts. ALDEHYDE AND KETONE REACTIONS (continued) • Examples: ALDEHYDE AND KETONE REACTIONS (continued) • The addition of alcohols to aldehydes produces an unstable hemiacetal intermediate. ALDEHYDE AND KETONE REACTIONS (continued) • Example: ALDEHYDE AND KETONE REACTIONS (continued) • Hemiacetal general formula: • Acetal general formula: ALDEHYDE AND KETONE REACTIONS (continued) • Hemiacetal example: • Acetal example: ALDEHYDE AND KETONE REACTIONS (continued) ALDEHYDE AND KETONE REACTIONS (continued) • Protonation of carbonyl oxygen • Alcohol oxygen bonds to carbonyl oxygen • Formation of hemiacetal and regeneration of acid catalyst ALDEHYDE AND KETONE REACTIONS (continued) • The addition of alcohol to ketones produces an unstable hemiketal intermediate. ALDEHYDE AND KETONE REACTIONS (continued) • Example: ALDEHYDE AND KETONE REACTIONS (continued) • Hemiketal general formula: • Ketal general formula: ALDEHYDE AND KETONE REACTIONS (continued) • Hemiketal example: • Ketal example: ALDEHYDE AND KETONE REACTIONS (continued) • Cyclic hemiacetals and hemiketals are more stable than open-chains and are important in carbohydrate chemistry. ALDEHYDE AND KETONE REACTIONS (continued) • Acetals and ketals are stable, but may be converted back to aldehydes and ketones through acid catalyzed hydrolysis. Hydrolysis is the cleavage of a bond by reaction with water. • Acetal hydrolysis: ALDEHYDE AND KETONE REACTIONS (continued) • Ketal hydrolysis: • Specific Example: ALDEHYDE AND KETONE REACTION MAP