* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Varicella-Zoster Virus
Survey
Document related concepts
Transmission (medicine) wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Diseases of poverty wikipedia , lookup
HIV and pregnancy wikipedia , lookup
Infection control wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Compartmental models in epidemiology wikipedia , lookup
Public health genomics wikipedia , lookup
Epidemiology wikipedia , lookup
Herpes simplex research wikipedia , lookup
Canine parvovirus wikipedia , lookup
Transcript
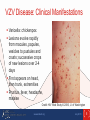
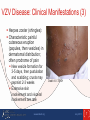
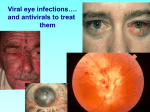
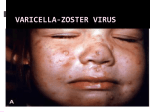
Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents Viral Infections Slide Set Prepared by the AETC National Coordinating Resource Center based on recommendations from the CDC, National Institutes of Health, and HIV Medicine Association/Infectious Diseases Society of America About This Presentation These slides were developed using recommendations published in July 2013. The intended audience is clinicians involved in the care of patients with HIV. Certain sections have been updated to reflect changes in the published guidelines. Users are cautioned that, because of the rapidly changing field of HIV care, this information could become out of date quickly. Finally, it is intended that these slides be used as prepared, without changes in either content or attribution. Users are asked to honor this intent. – AETC National Coordinating Resource Center http://www.aidsetc.org www.aidsetc.org July 2013 2 Varicella-Zoster Virus Epidemiology Clinical Manifestations Diagnosis Preventing Exposure & Disease Treatment Monitoring Preventing Recurrence Considerations in Pregnancy www.aidsetc.org July 2013 3 VZV Disease: Epidemiology Primary VZV = varicella (chickenpox) Reactivation of latent VZV results in herpes zoster (shingles) Lifetime risk 15-20%; highest incidence in immunocompromised and elderly Incidence >15-fold higher in HIV infected compared with general population Can occur at any CD4 count; highest frequency with CD4 count <200 cells/µL ART has not been shown to reduce incidence of zoster in adults Rates higher in period immediately after ART initiation www.aidsetc.org July 2013 4 VZV Disease: Clinical Manifestations Varicella: chickenpox Lesions evolve rapidly from macules, papules, vesicles to pustules and crusts; successive crops of new lesions over 2-4 days First appears on head, then trunk, extremities Pruritus, fever, headache, malaise Credit: HIV Web Study © 2006, U. of Washington www.aidsetc.org July 2013 5 VZV Disease: Clinical Manifestations (2) Varicella: chickenpox Illness may be severe in HIV infection Visceral dissemination, especially VZV pneumonitis, may occur www.aidsetc.org July 2013 6 VZV Disease: Clinical Manifestations (3) Herpes zoster (shingles): Characteristic painful cutaneous eruption (papules, then vesicles) in dermatomal distribution; often prodrome of pain New vesicle formation for 3-5 days, then pustulation and scabbing; crusts may peprsist 2-3 weeks Extensive skin involvement and visceral involvement are rare www.aidsetc.org Credit: © I-TECH July 2013 7 VZV Disease: Clinical Manifestations (4) Herpes zoster (shingles): Recurrence in 20-30% of HIV infected (same or different dermatome) Postherpetic neuralgia in 10-15% of HIV-infected persons Complications more common if CD4 count <200 cells/µL Neurologic syndromes: CNS vasculitis, multifocal leukoencephalitis, ventriculitis, myelitis and myeloradiculitis, optic neuritis, cranial nerve palsies, aseptic meningitis www.aidsetc.org July 2013 8 VZV Disease: Clinical Manifestations (5) Progressive outer retinal necrosis may be seen, almost exclusively with CD4 count <100 cells/µL Acute retinal necrosis: may occur at any CD4 count High rates of visual loss with both www.aidsetc.org July 2013 9 VZV Disease: Diagnosis Clinical diagnosis usually can be made, based on appearance of lesions Retrospective diagnosis of varicella by documenting seroconversion Atypical presentations may be seen in immunocompromised persons, may be hard to distinguish from disseminated zoster www.aidsetc.org July 2013 10 VZV Disease: Diagnosis (2) Definitive diagnosis: Viral culture, direct fluorescent antigen testing, or PCR from swabs from fresh lesion, or tissue biopsy, or scabs PCR is most sensitive and specific Histopathology and PCR of blood or fluids (eg, CSF, vitreous humor) www.aidsetc.org July 2013 11 VZV Disease: Preventing Exposure If susceptible (no history of varicella or zoster, not vaccinated, seronegative for VZV): avoid exposure to persons with varicella or zoster Susceptible household contacts of susceptible HIVinfected persons should be vaccinated www.aidsetc.org July 2013 12 VZV Disease: Preventing Disease Long-term prophylaxis with antiviral drugs is not recommended Vaccination to prevent primary infection Live attenuated varicella vaccine may be considered for HIV-infected, VZV-seronegative persons ≥8 years of age with CD4 count ≥200 cells/µL (2 doses, 3 months apart) No efficacy data in HIV-infected adults and adolescents, but safe and immunogenic in HIV-infected children with CD4 percentage ≥15% If vaccination results in disease caused by vaccine virus, treat with acyclovir Vaccination not recommended if CD4 <200 cells/µL www.aidsetc.org July 2013 13 VZV Disease: Preventing Disease (2) Postexposure prophylaxis to prevent primary infection: Varicella-zoster immune globulin (VariZIG) for VZVsusceptible HIV-infected children and adults Give as soon as possible (but ≤10 days) after close contact with person with active varicella or herpes zoster May consider short-term postexposure acyclovir or valacyclovir beginning 7-10 days after exposure (not studied in HIV infection) Acyclovir 800 mg PO 5 times/day for 5-7 days Valacyclovir 1 g PO TID for 5-7 days Postexposure varicella vaccination reduces risk of varicella in immunocompetent children; not studied in HIV infection www.aidsetc.org July 2013 14 VZV Disease: Preventing Disease (3) Vaccination after exposure If postexposure VariZIG has been given, wait ≥5 months before varicella vaccination If postexposure acyclovir has been given, wait ≥3 days before varicella vaccination www.aidsetc.org July 2013 15 VZV Disease: Treatment Varicella (chickenpox) Uncomplicated: Preferred Valacyclovir 1 g PO TID Famciclovir 500 mg PO TID Alternative Acyclovir 20 mg/kg up to maximum 800 mg PO 5 times daily Duration: 5-7 days Severe or complicated: Acyclovir 10-15 mg/kg IV Q8H for 7-10 days May switch to PO treatment as above after fever resolves, if no visceral involvement www.aidsetc.org July 2013 16 VZV Disease: Treatment (2) Herpes zoster Start treatment promptly if diagnosed within 1 week of rash onset, or any time before full crusting of lesions Local dermatomal zoster: Preferred Valacyclovir 1,000 mg TID Famciclovir 500 mg TID Alternative Acyclovir 800 mg PO 5 times per day Duration: 7-10 days (longer if lesions slow to resolve) www.aidsetc.org July 2013 17 VZV Disease: Treatment (3) Extensive cutaneous lesions or visceral involvement: Acyclovir 10-15 mg/kg IV Q8H, until clinical improvement After clinical improvement and no new cutaneous lesions, switch to PO therapy as above Duration: 10-14 days Adjunctive corticosteroid therapy not recommended (limited data in HIV infection) ART optimization is recommended for all VZV infections that are difficult to treat (eg, retinitis, encephalitis) www.aidsetc.org July 2013 18 VZV Disease: Treatment (4) Progressive outer retinal necrosis: Optimal therapy not defined; poor prognosis for vision preservation despite antiviral therapy Treatment should include at least one IV drug and at least one intravitreal anti-VZV drug, plus effective ART Ganciclovir 5 mg/kg IV Q12H and/or foscarnet 90 mg/kg IV Q12H PLUS ganciclovir 2 mg/0.05 mL intravitreal twice weekly and/or foscarnet 1.2 mg/0.05 mL intravitreal twice weekly Optimize ART Manage in conjunction with an experienced ophthalmologist www.aidsetc.org July 2013 19 VZV Disease: Treatment (5) Acute retinal necrosis: More responsive to antiviral therapy Acyclovir 10-15 mg/kg IV Q8H for 10-14 days, followed by valacyclovir 1 g PO TID for 6 weeks, PLUS ganciclovir 2 mg/0.05 mL intravitreal twice weekly x 1-2 doses Manage in conjunction with an experienced ophthalmologist www.aidsetc.org July 2013 20 VZV Disease: Starting ART Strongly consider ART initiation in patients with multiple recurrences of herpes zoster or a complication of VZV disease www.aidsetc.org July 2013 21 VZV Disease: Monitoring and Adverse Events IRIS: increased frequency of herpes zoster after initiation of ART, but clinical presentation and natural history are not different Valacyclovir, acyclovir: renal toxicity at high dosage Monitor renal function for patients on high-dose or prolonged therapy with IV acyclovir High-dose (8 grams/day) valacyclovir may cause thrombotic thrombocytopenic purpura/hemolytic uremic syndrome; not reported at lower dosages www.aidsetc.org July 2013 22 VZV Disease: Treatment Failure Suspect drug resistance if lesions do not start to resolve within 7-10 days of treatment initiation, or if they evolve to verrucous or atypical appearance Virus culture with susceptibility testing if virus is isolated If proven or suspected acyclovir resistance, treat with IV foscarnet (alternative: IV cidofovir) www.aidsetc.org July 2013 23 VZV Disease: Preventing Recurrence Efficacy of long-term antiviral prophylaxis to prevent recurrence of zoster not evaluated in HIV infection, not routinely recommended Herpes zoster vaccine: FDA approved for immunocompetent persons ≥50 years of age Contraindicated if CD4 count <200 cells/µL www.aidsetc.org July 2013 24 VZV Disease: Considerations in Pregnancy Postexposure prophylaxis: Recommended for VZV-susceptible HIV-infected pregnant women if close contact to person with active varicella or herpes zoster: Varicella-zoster immune globulin (VariZIG) Give as soon as possible (but ≤10 days) after exposure If acyclovir is used, obtain VZV serology and discontinue drug if patient is seropositive Varicella vaccine and herpes zoster vaccine should not be given during pregnancy www.aidsetc.org July 2013 25 VZV Disease: Considerations in Pregnancy (2) Diagnosis as in nonpregnant adults Treatment of varicella: Uncomplicated: PO acyclovir or valacyclovir Severe disease or VZV pneumonitis: hospitalize, IV acyclovir Treatment of zoster: PO acyclovir or valacyclovir www.aidsetc.org July 2013 26 VZV Disease: Considerations in Pregnancy (3) Risk of transmission to fetus if woman has primary VZV during first half of pregnancy Offer detailed ultrasound surveillance for signs of fetal congenital varicella VariZIG recommended to prevent complications in the mother (not known whether it prevents congenital varicella syndrome) Infants born to women with peripartum varicella (from 5 days before delivery until 2 days after) VariZIG to infant reduces severity and mortality of neonatal infection www.aidsetc.org July 2013 27 Websites to Access the Guidelines http://www.aidsetc.org http://aidsinfo.nih.gov www.aidsetc.org July 2013 28 About This Slide Set This presentation was prepared by Susa Coffey, MD, for the AETC National Resource Center in July 2013 and updated in May 2015. See the AETC NRC website for the most current version of this presentation: http://www.aidsetc.org www.aidsetc.org July 2013 29