* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Open Information Day 2011
Innate immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
DNA vaccination wikipedia , lookup
Neonatal infection wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Infection control wikipedia , lookup
Whooping cough wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Hepatitis B wikipedia , lookup
Immunocontraception wikipedia , lookup
HIV vaccine wikipedia , lookup
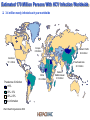
The HCV vaccine: cooperation in the shadow of the pyramids Antonella Folgori www.altaweb.it/hepacivac Estimated 170 Million Persons With HCV Infection Worldwide 3-4 million newly infected each year worldwide Europe 8.9 million Western Pacific 62.2 million Americas 13.1 million Southeast Asia 32.3 million Africa 31.9 million Prevalence of infection >10% 2.5%–10% 0.5%–2.5% No information World Health Organization 2008 Eastern Mediterranean 21.3 million Egypt has the highest prevalence of HCV infection in the world • 15% anti-HCV positive among adult rural Nile Delta inhabitants (EDHS, 2009; Strickland 2006; Frank et al, 2000; Abdel-Aziz et al, 2000; Waked et al, 1995) Europe 8.9 million Western Pacific 62.2 million Americas 13.1 million Southeast Asia 32.3 million Africa 31.9 million Prevalence of infection >10% 2.5%–10% 0.5%–2.5% No information Eastern Mediterranean 21.3 million HCV infection and medical needs • Infection is usually asymptomatic • 80% of infected individuals become chronic • HCV is a common cause of liver disease and represents a major threat to the health of many people around the world • Interferon based treatment is effective in many people but it has extensive side effects and it is very expensive • The addition of new antiviral agents will improve virological response rates and decrease the duration of treatment but will likely have further side effects and additional costs • Large population of undiagnosed and untreated persons Historically, vaccination is needed to eradicate infectious diseases - not drugs 19 A new vaccination approach A classical vaccine triggers the production of antibodies which recognize the surface of the virus In the case of HCV this structural part changes constantly Virus Target cell Antibodies The new vaccination approach relies on the generation of «killer» T lymphocytes that reacts to the presence of infectious agents and destroy the infected cells In the case of HCV T-cell response plays a critical role in viral control in early infection Virus Infected target cell Cytotoxic T lymphocytes Genetic vaccines The best way to elicit a T cell response is to deliver the gene coding for parts of the infectious agents The gene is used as a source of antigen and the muscle as a “bioreactor” to produce the corresponding protein Recombinant viral vectors Antigenic peptide Antigen encoding gene MHC Immune system activation (T cells & Abs) Antigen HCV vaccine: an international team of researchers is rising to the challenge 12 project partners from 7 countries: Belgium, Germany, Italy, the Netherlands, Poland, UK and Egypt Development of a prophylactic HCV vaccine – to eradicate infection Use the same approach to treat infected patients – to improve on SOC HCV Vaccine for Prophylaxys and Therapy: The HEPACIVAC Strategy Surface antigens HCV E1E2 Subunit vaccine +++ Antibodies +/- T cells Genetic vaccine 2 Non Structural antigens NS Genetic vaccine 1 +++ T cells NO GO Preclinical evaluation in mice and non human primates Manufacturing in GMP 01 Go to Clinical trials Has the effort been successful? What next? 06 Achievements - I Preclinical testing of the vaccine: safety, tolerability and strong immunogenicity demonstrated in animal models Standardization of the procedures for pre-clinical and clinical trials for HCV Transfer of knowledge (and reagents!) between participant groups in particular from Europe to Egypt Evaluation of HCV incidence and cell mediated immune responses among Egyptian HCW: a preparedness study for a future Phase II trial of the HCV prophylactic vaccine in Egypt Achievements - II Two Clinical studies in healthy volunteers for safety, dose and administration regimens optimization – completed: vaccine very safe & highly immunogenic Three Clinical studies in in chronically infected patients with and without the gold standard therapy – in progress; interim data showed that the vaccine is safe and immunogenic Post-HEPACIVAC plans: translate the results into effective approaches for prevention and therapy of HCV Phase II efficacy study of the HEPACIVAC vaccine in high risk individuals • • Established strategic alliance with UCSF and JH in the US who study target populations with high and stable incidence of HCV infection Support from NIH Clinical studies in Egypt Collaborative research required: Tight coordination Networking Exchange of experimental data and discussion / teleconference meetings among «working groups» Supervision of the activities so as to ensure the progression of the project towards the delivery of the objectives Open discussion during annual meetings Cairo, 2009 Acknowledgments:


















![[635] Amniocentesis in Women Infected with Hepatitis C Virus](http://s1.studyres.com/store/data/009708012_1-d1a537c5873ff9b4d2a68359d3cf01a2-150x150.png)







