* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Leptin Signaling in the Nucleus Tractus Solitarii
Aging brain wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroplasticity wikipedia , lookup
History of neuroimaging wikipedia , lookup
Brain Rules wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuropsychology wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Selfish brain theory wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neural engineering wikipedia , lookup
Development of the nervous system wikipedia , lookup
Optogenetics wikipedia , lookup
Synaptic gating wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroanatomy wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Microneurography wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
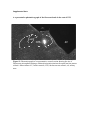
Leptin Signaling in the Nucleus Tractus Solitarii Increases Sympathetic Nerve Activity to the Kidney Allyn L. Mark, Khristofor Agassandian, Donald A. Morgan, Xuebo Liu, Martin D. Cassell, Kamal Rahmouni Downloaded from http://hyper.ahajournals.org/ by guest on April 29, 2017 Abstract—The hypothalamic arcuate nucleus was initially regarded as the principal site of leptin action, but there is increasing evidence for functional leptin receptors in extrahypothalamic sites, including the nucleus tractus solitarii (NTS). We demonstrated previously that arcuate injection of leptin increases sympathetic nerve activity (SNA) to brown adipose tissue and kidney. In this study, we tested the hypothesis that leptin signaling in the NTS affects sympathetic neural outflow. Using a stereotaxic device in anesthetized rats, we microinjected leptin (0.25 to 1.00 g) or saline into the NTS while recording SNA to kidney and brown adipose tissue. Microinjection of leptin into the commissural and medial subnuclei of the caudal NTS at the level of the area postrema in Sprague-Dawley rats produced a dose-related increase in renal SNA (⫹112⫾15% with leptin 1 g; n⫽7; P⬍0.001) but did not increase SNA to brown adipose tissue (⫺15⫾12%; P value not significant). This effect depended on intact functional leptin receptors, because it was not observed in Zucker obese rats that have a missense mutation in the leptin receptor. Rostral NTS injection of leptin failed to increase SNA, indicating that leptin signaling in the NTS is probably confined to the caudal NTS at the level of the area postrema. In summary, this study demonstrates that leptin signaling in the caudal NTS increases SNA to the kidney but not to the brown adipose tissue. The study strengthens the concept of a distributed brain network of leptin action and demonstrates that these distributed brain sites can mediate contrasting sympathetic responses to leptin. (Hypertension. 2009;53[part 2]:375-380.) Key Words: leptin 䡲 nucleus tractus solitarii 䡲 sympathetic nerve activity 䡲 kidney 䡲 brown adipose tissue L eptin is an adipocyte-derived hormone that plays a critical role in the regulation of energy homeostasis. The hypothalamic arcuate nucleus was initially regarded as the principal site of leptin action, but there is increasing evidence for a distributed brain network of leptin action.1 Among these distributed brain sites, there is increasing evidence for functional leptin receptors in the nucleus tractus solitarii (NTS), albeit in lower levels than in the arcuate nucleus. Functional leptin receptor mRNA is expressed in the NTS of mice and rats.2– 4 Peripheral administration of leptin increases phosphorylated signal transducers and activators of transcription 34,5 and c-fos6,7 immunoreactivities in the NTS. Proopiomelanocortin neurons, which are prominent in mediating leptin action in the arcuate nucleus, also occur in the NTS.8 –10 There is controversy centering on whether these NTS proopiomelanocortin neurons do10 or do not1,11 mediate some of the NTS leptin action, but there is agreement that there are leptin-responsive neurons in the NTS.1–7,9 –12 For example, Grill et al3 demonstrated that a low dose of leptin injected into the dorsal vagal complex including the NTS decreased food intake and body weight in rats. In addition, a number of NTS neurons that are activated by gastric disten- sion are also activated by leptin,12,13 and leptin acts in the hindbrain to potentiate the satiety response to gastric distension.12 In addition to effects on appetite and body weight, leptin increases sympathetic nerve activity (SNA).14 –17 With systemic,15 cerebroventricular,7,16 or intra-arcuate administration of leptin,17 there are increases in SNA to brown adipose tissue (BAT), which is involved in thermogenic metabolism, and to the kidney, which is involved in cardiovascular regulation. Because the NTS is critically involved in sympathetic regulation and given the emerging evidence for leptin signaling in the NTS, we tested the hypothesis that leptin receptor activation in the NTS regulates sympathetic neural activity. Methods Animals Male Sprague-Dawley rats and Zucker obese and lean rats (Harlan Sprague-Dawley), age 14 to 16 weeks, were studied. Rats were housed in a temperature-controlled room with a 12:12 hour lightdark cycle. Standard laboratory rat chow and tap water were provided ad libitum. The studies and procedures were approved by the University of Iowa Animal Research Committee. All of the Received October 3, 2008; first decision October 24, 2008; revision accepted November 25, 2008. From the Departments of Internal Medicine (A.L.M., D.A.M., X.L., K.R.) and Anatomy and Cell Biology (K.A., M.D.C.) and Center on Functional Genomics of Hypertension (A.L.M., K.A., D.A.M., X.L., M.D.C., K.R.), Carver College of Medicine, University of Iowa, Iowa City. Correspondence to Allyn L. Mark, 3187 MERF, University of Iowa Carver College of Medicine, Iowa City, IA 52242-1101. E-mail [email protected] © 2009 American Heart Association, Inc. Hypertension is available at http://hyper.ahajournals.org DOI: 10.1161/HYPERTENSIONAHA.108.124255 375 376 Hypertension February 2009, Part II studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental Preparation Rats were anesthetized with IP ketamine (91.0 mg/kg) and xylazine (9.1 mg/kg). After ketamine and xylazine anesthesia and insertion of a venous catheter, ␣-chloralose 50 mg/kg was administered intravenously in 0.5 mL over 45 minutes followed by 25 mg/kg per hour in 0.5 mL/h. Arterial pressure and heart rate were monitored with a catheter inserted into a caudal artery and a pressure transducer connected to a computer. The trachea was cannulated, and each rat was allowed to breathe oxygen-enriched air spontaneously. Rectal temperature was maintained at 37.5°C using a temperaturecontrolled surgical table and lamp. The animal was instrumented for sympathetic nerve recordings as described below and was then placed in a stereotaxic frame (David Kopf Instruments) and prepared for stereotaxic microinjections into the NTS as described below. Sympathetic Nerve Recordings Downloaded from http://hyper.ahajournals.org/ by guest on April 29, 2017 After the induction of anesthesia, we obtained simultaneous multifiber recordings of SNA from a nerve to the kidney and to the BAT. The left kidney was exposed through a retroperitoneal flank incision. Using a dissecting microscope, a renal nerve was carefully dissected free and placed on a bipolar 36-gauge platinum-iridium electrode. When an optimal recording of renal SNA was obtained, the electrode was covered with silicone gel (Kwik-Sil, World Precision Instruments, Inc). Interscapular BAT was then exposed through a nape incision. A nerve fiber innervating BAT was identified, placed on the bipolar electrode, and secured with silicone gel for recordings of SNA to BAT. Each electrode was attached to a high-impedance probe (HIP-511, Grass Instruments), and the nerve signal was amplified 105 times with a Grass P5 AC preamplifier. After amplification, the nerve signal was filtered at a 100- and 1000-Hz cutoff with a nerve traffic analysis system (Model B600c, University of Iowa Bioengineering). The nerve signal was then routed to an oscilloscope (Model 54501A, Hewlett-Packard) for monitoring the quality of the sympathetic nerve recording and to a resetting voltage integrator (Model B600c, University of Iowa Bioengineering). Brain Microinjections Once the surgery for SNA recordings was complete, the head of the rat was placed in a stereotaxic frame. The head of the rat was flexed ⬇30° in the stereotaxic device to level the caudal brain stem. For microinjections into the caudal NTS, after surgical exposure, the pipette was placed in the brain stem 0.4 to 0.5 mm rostral to the calamus scriptorius, 0.4 to 0.6 mm lateral to the midline, and 0.5 to 0.7 mm beneath the surface of the brain stem. After injections, the pipette was left in place for 5 minutes and then removed. For microinjection into the rostral NTS, the pipette was placed in the brain stem 0.8 to 1.0 mm rostral to the calamus scriptorius, 0.8 to 1.0 mm lateral to the midline, and 0.5 to 0.7 mm beneath the surface of the brain stem. We mixed fluorescent beads (0.2% FluoSphere, 1 m, blue fluorescent, Invitrogen) with leptin and vehicle for each injection to assess the accuracy of the injection site. At the end of each experiment, the animal was perfused with 4.0% paraformaldehyde and 0.5% glutaraldehyde. The brain was sectioned, and brain sections were reviewed by an investigator (M.D.C.) who was blinded to the experimental protocol. Only experiments in which the injection site was verified were included. Experimental Protocols After surgical preparation, rats were allowed to stabilize for 15 to 20 minutes. Baseline recordings of sympathetic nerve activities, arterial pressure, and heart rate were collected twice during a 10-minute control period and averaged for a single value for the control period. In the parent protocol, Sprague-Dawley rats received either vehicle (saline, 50 nL) or recombinant mouse leptin (0.25, 0.50, or 1.00 g in 50 nL saline; R&D Systems, Inc) into the left caudal NTS. Baseline 5 hrs after leptin (0.5 μg) into NTS Renal SNA BAT SNA 15 sec Figure 1. Segments of record showing neurograms of renal and BAT SNA at baseline and 5 hours after injection of leptin (0.5 g) into the caudal NTS. NTS leptin increased renal but not BAT SNA. In 6 experiments, we injected leptin 0.5 g in 50 nL of saline into both the left and right caudal NTS. In the above and subsequent protocols, recordings of SNA, arterial pressure, and heart rate were obtained every 15 minutes for 5 hours after the injections. To determine whether the responses were leptin receptor mediated, we performed experiments in Zucker obese rats that have a missense mutation in the leptin receptor18 to determine whether the effects of leptin are receptor mediated. We injected leptin (0.5 g in 50 nL of saline) into the left caudal NTS of Zucker obese and lean rats. To determine whether other regions of the NTS beside the caudal NTS mediate leptin signaling of SNA, we injected leptin (0.5 g in 50 nL of saline) into the left rostral NTS in Sprague-Dawley rats. Data Analysis To ensure that electronic noise was excluded from the measurement of SNA, each SNA recording was corrected for postmortem background activity. Sympathetic nerve responses are expressed as the percentage of change from baseline values. Results are presented as means⫾SEMs. Data were analyzed using unpaired t test or 1- or 2-way ANOVA. Time-course responses were analyzed using repeated-measures 2-way ANOVA. Fisher least significant difference posthoc test was used for pairwise comparisons, as appropriate. A value of P⬍0.05 was considered significant. Results Responses to Caudal NTS Injections of Leptin Injection of leptin (0.25 to 1.00 g in 50 nL of saline) into the caudal NTS of Sprague-Dawley rats resulted in a dose-related increase in renal SNA (Figures 1 and 2). Bilateral injection of 0.50 g of leptin into the caudal NTS produced an increase in renal SNA (⫹85⫾13%; n⫽5) that did not differ (P value not significant) from that seen with injection of 1.00 g of leptin unilaterally (⫹112⫾15%). In contrast to the effects on renal SNA, unilateral caudal NTS injection of leptin did not increase SNA to BAT (Figures 1 and 2). Bilateral caudal NTS injection of 0.5 g of leptin also failed to increase SNA to BAT (⫹2⫾13%; n⫽5). Caudal NTS injection of vehicle (50 nL of saline) did not alter either renal or BAT SNA (Figure 2A through 2D). To ascertain that the lack of increase in SNA to BAT was not caused by a lack of responsiveness of the preparation, we studied responses to cooling at the end of 12 experiments in which NTS leptin injection failed to increase SNA to BAT. Cooling the rats from 37.5° to 30.0°C increased SNA to BAT by ⫹122⫾41% (P⬍0.05). Cooling increased SNA to BAT in each of these 12 experiments. These results indicate that the failure of leptin to increase SNA to BAT cannot be explained by a lack of responsiveness of the preparation. Mark et al Vehicle Leptin (1 μg) 100 * ** * C * * Δ Renal SNA (%) Δ Renal SNA (%) 150 * * A Leptin Signaling in the Nucleus Tractus Solitarii 50 0 -50 0 60 120 180 240 300 150 377 * 100 * 50 0 Leptin (μg) 0 0.25 0.5 1.0 0 0.25 0.5 1.0 Time (min) 150 D 100 60 Δ BAT SNA (%) Vehicle Leptin (1 μg) 50 0 30 0 -50 0 60 120 180 240 300 Time (min) -30 Leptin (μg) Figure 2. Effects of leptin injection into the caudal NTS on renal and BAT SNA in rats. The time course of renal and BAT SNA responses to NTS injection of vehicle (50 nL of saline) or leptin (1.0 g in 50 nL of saline) is shown in the left panels (A and B). Caudal NTS injection of leptin, 1.0 g, produced a significant increase in renal (P⬍0.001) but not BAT (P value not significant) SNA. Summary data for percentage of change in renal and BAT SNA 5 hours after NTS injection of vehicle (50 nL of saline) or leptin (0.25, 0.50, or 1.00 g in 50 nL of saline) are shown in the right panels (C and D). Vehicle (labeled leptin 0) had no effect. Caudal NTS injection of leptin produced a dose-related increase in renal SNA (P⬍0.001) but failed to increase BAT SNA (P value not significant). Data are means⫾SEMs of n⫽4 to 7 per group. *P⬍0.05 vs vehicle. The location of the injection sites confirmed to be within the caudal NTS is shown in Figure 4. The injections were distributed in the lateral part of the commissural subnucleus and the dorsomedial part of the medial subnucleus. There was no notable difference in the responses to leptin injection into the lateral part of the commissural subnucleus versus the dorsomedial part of the medial subnucleus. As shown in Figure 3, mean arterial pressure increased significantly (P⬍0.05 versus vehicle) with the high dose of leptin but not with the low or middle dose when compared with responses to vehicle. Baseline mean arterial pressure was 87⫾11, 95⫾4, 86⫾4, and 94⫾2 mm Hg in the vehicle and 0.25-, 0.50-, and 1.00-g leptin groups, respectively. Heart rate increased in both the vehicle and leptin groups, but the increases did not differ between groups (P value not significant). For example, heart rate increased from 229⫾16 to 299⫾12 bpm in the vehicle group and from 242⫾13 to 302⫾8 bpm in the leptin 0.50-g group. Δ MAP (mmHg) 25 Vehicle Leptin (1 μg) 15 To determine whether the SNA responses to caudal NTS leptin are receptor mediated, we compared effects of caudal * ** * * * ** * * * 5 -5 -15 B * 25 Δ MAP (mmHg) A Responses to NTS Injection of Leptin in Zucker Lean and Obese Rats * Downloaded from http://hyper.ahajournals.org/ by guest on April 29, 2017 Δ BAT SNA (%) B 15 5 -5 -15 -25 0 45 90 135 180 225 Time (min) 270 315 -25 Leptin (μg) 0 0.25 0.5 1.0 Figure 3. Effects of leptin injected into the caudal NTS on arterial pressure. A, Time course of mean arterial pressure (MAP) responses to NTS injection of vehicle or leptin (1.0 g). B, Summary data for changes in MAP 5 hours after NTS injection of vehicle (50 nL of saline shown as leptin 0) or leptin (0.25, 0.50, and 1.0 g). Data are means⫾SEMs of n⫽4 to 7 per group. *P⬍0.05 vs vehicle. 378 Hypertension February 2009, Part II Sprague-Dawley rats failed to increase SNA to either BAT (⫺21⫾17%) or kidney (⫹5⫾25%). A representative photomicrograph of the florescent beads in the rostral NTS is shown in Figure S1 (available in the online data supplement at http://hyper.ahajournals.org). The lack of SNA response to rostral NTS leptin is consistent with previous reports that leptin receptor signaling in the NTS is confined to the caudal NTS.1,10,11 AP cNTS sol iNTS cc mNTS DMX Discussion 1 mm NTS injection of leptin (0.5 g in 50 nL of saline) between Zucker obese rats that have a missense mutation in the leptin receptor18 and lean controls. As shown in Figure 5A, injection of leptin into the caudal NTS increased renal SNA in the Zucker lean but not in the Zucker obese rats. NTS injection of leptin failed to increase BAT SNA in either Zucker lean or obese rats (Figure 5B). These findings support the conclusion that the renal SNA responses to caudal NTS in the SpragueDawley and Zucker lean control rats are dependent on an intact leptin receptor. Responses to Rostral NTS Injections of Leptin We also studied responses to leptin injection into the rostral NTS to determine whether this region of the NTS might participate in leptin signaling of sympathetic outflow, because viral tracing has indicated that the rostral and intermediate NTS, but not the caudal NTS, are polysynaptically connected with BAT.19 In the current study, injection of leptin (0.5 g in 50 nL of saline) into the rostral NTS in B 150 120 100 * 50 0 ZL ZO Δ BAT SNA (%) A Δ Renal SNA (%) Downloaded from http://hyper.ahajournals.org/ by guest on April 29, 2017 Figure 4. Schematic diagram of the NTS showing the sites of leptin injection assessed from the location of fluorescent microspheres coinjected with leptin. The injections were localized in the lateral part of the commissural subnucleus and the dorsomedial part of the medial subnucleus of the NTS. The size of each marker reflects the borders of the deposit of microspheres. cNTS indicates commissural subnucleus; iNTS, intermediate subnucleus; mNTS, medial subnucleus; DMX, dorsal vagal motor nucleus; AP, area postrema; sol, solitary tract. 70 20 -30 ZL ZO Figure 5. Effects of 0.5 g of leptin injected into the caudal NTS on renal and BAT SNA in leptin receptor mutant Zucker obese (ZO) and control Zucker lean (ZL) rats. A, Comparison of renal SNA response to leptin injection into the NTS between the Zucker obese and lean control rats. B, Comparison of BAT SNA response to leptin injection into the NTS between the Zucker obese and lean control rats. Data are means⫾SEMs of n⫽4 to 7 per group. *P⬍0.05 vs lean controls. The major new findings from this study are as follows: (1) leptin receptor signaling in the NTS increases SNA to kidney but not to BAT; (2) sympathoactivation to microinjection of leptin into the NTS was associated with an increase in arterial pressure; and (3) the renal sympathetic nerve responses to NTS leptin appear to emanate from the caudal region of the NTS at the level of the area postrema. Leptin signaling in the NTS produces a strikingly different pattern of sympathetic responses than that emanating from leptin signaling in the hypothalamic arcuate nucleus in our previous study.17 The current study adds to evidence for functional leptin receptors in the NTS and thereby strengthens the concept of a distributed brain network of leptin action. The results further demonstrate that these distributed brain sites can mediate contrasting responses to leptin. As discussed in the introduction, our study was prompted by emerging evidence for functionally significant leptin receptors in the NTS.1–13 NTS injection of leptin failed to increase SNA in Zucker obese rats that have a missense mutation in the leptin receptor that renders the leptin receptor dysfunctional, indicating that the renal SNA responses to NTS leptin in the Sprague-Dawley and Zucker lean control rats were leptin receptor mediated. Injection of leptin into the caudal NTS increased renal SNA in both Sprague-Dawley and Zucker lean or wild-type rats but failed to increase SNA to BAT. We considered 2 possible explanations for the failure of caudal NTS to increase SNA to BAT. The first was a nonspecific lack of responsiveness of BAT SNA in our preparation. This was excluded by demonstrating that cooling the rats, which is a stimulus to thermogenic metabolism, increased SNA to BAT. In addition, in our previous report, using the same approach as in the current study, microinjection of leptin into the hypothalamic arcuate nucleus increased both BAT and renal SNA.17 Second, we considered the possibility that the rostral, as opposed to the caudal, NTS region might regulate SNA to BAT, but injection of leptin into the rostral NTS failed to increase SNA to either the kidney or BAT. Injections of pseudorabies virus, a transneuronal tracer, into interscapular BAT result in early infection of the rostral and intermediate NTS, and many neurons so infected are responsive to cold.19 However, injection of leptin into the rostral NTS failed to increase SNA to either the kidney or BAT. Our data, therefore, suggest that leptin receptor activation in the NTS does not initiate sympathetic activation to thermogenic BAT. Our studies do not exclude a role for the NTS in the neural pathways mediating leptin-induced increases in SNA to BAT, but the findings suggest that leptin signaling in the NTS does not trigger sympathetic activation to BAT. This Mark et al Downloaded from http://hyper.ahajournals.org/ by guest on April 29, 2017 is supported by the findings that increased leptin transgenic expression in the dorsal vagal complex, including the NTS, failed to affect uncoupling protein-1 mRNA expression in the BAT.20 The finding that caudal NTS leptin increased renal SNA, whereas rostral NTS injection of leptin failed to increase SNA, supports previous suggestions1,10,11 that leptin signaling in the NTS occurs primarily in the caudal NTS at the level of the area postrema. Our injections into the caudal NTS were clustered in the lateral part of the commissural subnucleus and the dorsomedial part of the medial subnucleus. There were no notable differences in the renal SNA responses to leptin in these 2 areas. Anatomic studies suggest that the caudal NTS may be indirectly connected with postganglionic sympathetic neurons innervating the kidney,21 and there is evidence that visceral afferents from the kidney ultimately end in the commissural NTS.22 We cannot completely exclude the possibility that the area postrema and/or dorsal motor nucleus of the vagus contributed to the renal SNA responses to the injection of leptin in the caudal NTS. The vast majority of the injection sites into the caudal NTS were centered lateral to the area postrema and dorsal to the dorsal motor nucleus of the vagus (Figure 4), but some leptin may have diffused, albeit at decreasing concentration, into the area postrema or the nucleus of the vagus. Nevertheless, given the distribution of the center of the injection sites in the caudal NTS, the most likely interpretation of our data is that the responses emanated mainly from the commissural and medial subnuclei of the caudal NTS. From these studies, we cannot identify the NTS neurons activated by leptin to increase SNA. NTS leptin increased renal SNA (which is under baroreceptor control) but not SNA to BAT (which is not under baroreceptor control). Our NTS injection sites include neurons in receipt of baroreceptor afferents.23 These observations suggest that leptin could be acting in the NTS to increase renal SNA by acting on baroreceptor neurons, but our NTS injection sites also include neurons subserving visceral afferents,24 and NTS neurons that are activated by gastric distension are also activated by leptin.12,13 Caudal NTS injection of leptin increased arterial pressure. However, this effect was significant only with the highest dose of leptin. This is consistent with the modest pressor action of leptin, particularly in acute studies.16,17 The pressor action of caudal NTS injection of leptin may be attributable to sympathetically mediated vasoconstriction or a consequence of the renal sympathetic antinatriuretic actions of leptin. However, additional studies are needed to test this hypothesis. Perspectives A striking and surprising finding was the contrast between the pattern of sympathetic activation with NTS leptin in this study and the pattern of sympathetic responses to arcuate administration of leptin in our previous study.17 NTS injection of leptin increased renal SNA but failed to increase SNA to BAT. In contrast, in our previous study, arcuate injection of leptin (in the same dose injected into Leptin Signaling in the Nucleus Tractus Solitarii 379 the NTS) increased SNA to both BAT and kidney with greater increases in BAT SNA.17 The contrasting pattern of responses to NTS versus arcuate leptin suggests that the responses to NTS leptin are not emanating from the hypothalamic arcuate nucleus. The finding that both NTS and arcuate leptin trigger SNA strengthens the concept of a distributed brain network of leptin action. The contrasting responses to NTS versus arcuate leptin suggest that these distributed brain sites can mediate qualitatively distinct leptin actions. Our study extends the understanding of the brain regions involved in the sympathetic neural actions of leptin and the concept of a distributed brain network of leptin action. Acknowledgments We acknowledge the assistance of David Dellsperger in data management and the secretarial assistance of Jan Rogers. Sources of Funding This work was supported by the National Institutes of Health grant HL084207 to A.L.M. and K.R. and by American Heart Association grant 0530274N to K.R. Disclosures None. References 1. Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity. 2006;14:216S–221S. 2. Mercer JH, Moar KM, Hoggard N. Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology. 1998; 139:29 –34. 3. Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239 –246. 4. Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin’s action in the central nervous system. Endocrinology. 2002;143:3498 –3504. 5. Munzberg H, Huo L, Nillni E, Hollenberg AN, Bjørbæk C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. 6. Elmquist JK, Ahima RS, Maratos-Flier E, Flier J, Saper C. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839 – 842. 7. Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. 8. Bronstein DM, Schafer MK-H, Watson SJ, Akil H. Evidence that -endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 1992;587:269 –275. 9. Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. 10. Ellacott KLJ, Halatchev IG, Cone RD. Characterization of leptinresponsive neurons in the caudal brainstem. Endocrinology. 2006;147: 3190 –3195. 11. Huo L, Grill HJ, Bjørbæk C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. 12. Huo L, Maeng L, Bjørbæk C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology. 2007;148:2189 –2197. 13. Schwartz GJ, Moran TH. Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779 –3784. 380 Hypertension February 2009, Part II 14. Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. 15. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptormediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270 –278. 16. Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity in normal rats. Diabetes. 1997;46: 2040 –2043. 17. Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49: 647– 652. 18. Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CT, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18 –19. 19. Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to 20. 21. 22. 23. 24. interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. Boghossian S, Lecklin A, Dube MG, Kalra PS, Kalra SP. Increased leptin expression in the dorsal vagal complex suppresses adiposity without affecting energy intake and metabolic hormones. Obesity. 2006;14: 1003–1009. Sly DJ, Colvill L, McKinley MJ, Oldfield BJ. Identification of neural projections from the forebrain to the kidney using the virus pseudorabies. J Autonom Nerv Sys. 1999;77:73– 82. Weiss ML, Chowdhury SI. The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res. 1998;812:227–241. Ciriello J. Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci Lett. 1983;36:37– 42. Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J Comp Neurol. 1991;304:261–274. Downloaded from http://hyper.ahajournals.org/ by guest on April 29, 2017 Leptin Signaling in the Nucleus Tractus Solitarii Increases Sympathetic Nerve Activity to the Kidney Allyn L. Mark, Khristofor Agassandian, Donald A. Morgan, Xuebo Liu, Martin D. Cassell and Kamal Rahmouni Downloaded from http://hyper.ahajournals.org/ by guest on April 29, 2017 Hypertension. 2009;53:375-380; originally published online December 22, 2008; doi: 10.1161/HYPERTENSIONAHA.108.124255 Hypertension is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2008 American Heart Association, Inc. All rights reserved. Print ISSN: 0194-911X. Online ISSN: 1524-4563 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://hyper.ahajournals.org/content/53/2/375 Data Supplement (unedited) at: http://hyper.ahajournals.org/content/suppl/2008/12/22/HYPERTENSIONAHA.108.124255.DC1 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Hypertension can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Hypertension is online at: http://hyper.ahajournals.org//subscriptions/ Online supplement LEPTIN SIGNALING IN THE NUCLEUS TRACTUS SOLITARII INCREASES SYMPATHETIC NERVE ACTIVITY TO THE KIDNEY Allyn L. Mark(1,3), Khristofor Agassandian(2,3), Donald A. Morgan(1,3), Xuebo Liu (1,3), Martin D Cassell(2.3), Kamal Rahmouni(1,3) Departments of Internal Medicine(1) and Anatomy and Cell Biology(2) and Center on Functional Genomics of Hypertension(3), Carver College of Medicine, University of Iowa, Iowa City, Iowa Short title: Leptin signaling in the nucleus tractus solitarii. Correspondence to: Allyn L. Mark, M.D. 3187 MERF University of Iowa Carver College of Medicine Iowa City, IA, 52242-1101, USA Email: [email protected] Tel: 319 353 5674 Fax: 319 353 5350 Supplemental data: A representative photomicrograph of the florescent beads in the rostral NTS sol NTS 4V Figure S1. Photomicrograph of a representative coronal section showing the site of fluorescent microspheres during a unilateral microinjection into the rostral nucleus tractus solitarii. Abbreviations: 4V, fourth ventricle; NTS, nucleus tractus solitarii; sol, solitary tract.