* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cytogenetic Analysis Shows that the Unusually Large Chromosome
Point mutation wikipedia , lookup
Genomic library wikipedia , lookup
Comparative genomic hybridization wikipedia , lookup
Medical genetics wikipedia , lookup
Designer baby wikipedia , lookup
Hybrid (biology) wikipedia , lookup
Polymorphism (biology) wikipedia , lookup
Pathogenomics wikipedia , lookup
Microevolution wikipedia , lookup
Genomic imprinting wikipedia , lookup
DNA supercoil wikipedia , lookup
Biology and sexual orientation wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene expression programming wikipedia , lookup
Segmental Duplication on the Human Y Chromosome wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genome (book) wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Y chromosome wikipedia , lookup
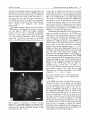
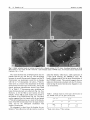
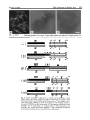
Hereditas 133: 95- 103 (2000) Cytogenetic analysis shows that the unusually large chromosome in the sex-limited p B silkworm (Bombyx mori) strain consists of three chromosomes NOBUHIKO TANAKA' , TAKESHI YOKOYAMA', HIROAKI ABE', KOZO TSUCHIDA', OSAMU NINAGI' and TOSHIKAZU OSHIKI' Department of Biological Production, Faculty o j Agriculture, Tokyo University of Agriculture and Technology, Saiwai-cho 3-5-8, Fuchu, Tokyo 183-8509, Japan National Institute of Infectious Diseases, Toyama 1-23-1, Shinjuku-ku, Tokyo 162-8640, Japan Tanaka, N., Yokoyama, T., Abe, H., Tsuchida, K., Ninagi, 0. and Oshiki, T. 2000. Cytogenetic analysis shows that the unusually large chromosome in the sex-limited p B silkworm (Bombyx mori) strain consists of three chromosomes. Hereditas 133: 95-103. Lund, Sweden. ISSN 0018-0661. Received August 7, 2000. Accepted October 30, 2000 ~ We have discovered an inordinately large chromosome pair at the pachytene stage in the oocyte of the sex-limited p B (black larval marking) silkworm (Bombyx mori) strain (TWPB). We have analyzed the composition and arrangement of this large chromosome. A genetic linkage analysis shows that the large chromosome is made up of the W chromosome, the second chromosome fragment (p fragment), and the fifth chromosome (linkage group) containing at least the region from map position 0.0 to 40.8. We also observed a sex heterochromatin body (SB) that we deduced to be made up of condensed W chromosomes. The number of SBs in each female nucleus among the sucking stomach cells of the TWPB strain was variable. Evidently, the W chromosome of the TWPB strain is attached to another chromosome. The composition of the W chromosome, the second chromosome fragment, and the fifth chromosome was studied through linkage analysis for these three chromosomes. We used two strains derived from the TWPB strain, the sex-limited p M (moricaud barVal marking)-hke (TWPML) and the autosomal p M-like (TSPML). The results show that the TWPML strain originates through a detachment of the fifth chromosome from the large chromosome of the TWPB strain, and the TSPML strain originates through a detachment of the W chromosome from that. Accordingly, the large chromosome of the TWPB strain is arranged in the order W chromosome-second chromosome fragment-fifth chromosome. Nobuhiko Tanaka, Laboratory of Sericulturul Science, Depariment of Biological Production, Faculty of Agriculture, Tokyo University of Agriculture and Technology, Saiwai-cho 3 - 5 8 , Fuchu, Tokyo 183-8509, Japun. E-mail: [email protected] TANAKA(19 16) showed that females of the silkworm, Bombyx mori, are ZW, and males are ZZ. The female sex is determined through a single W chromosome. This was confirmed by the observation of polyploids (HASIMOTO1933; KAWAGUCH~ 1934), a sex chromosome non-disjunction strain (TANAKA1939), and sexlimited strains (TAZIMA1944). Chromosomal constitutive aberrations such as translocation, duplication, deletion, and attachment have been frequently found in B. mori (TAKASAKI 1952; FUJIIet al. 1998). Strains that have an autosoma1 fragment having a dominant gene for egg color, larval marking, and blood color translocated on the W chromosome are useful because sex can easily be determined through the sex-limited expression of characteristics (TAZIMA1941; TAZIMAet al. 1951; HASIMOTO1948; KIMURAet al. 1971). TAZIMA (1941) found the sex-limited p s u (sable larval marking) strain. This strain had a translocation between the W and the second chromosome having a mutant allele in the p locus, which controls larval markings. TAZIMA(1942, 1943, 1944) irradiated this sex-limited strain with X rays and obtained a sex-limited normal marking ( + ") strain, which had eliminated the exces- sive part of the translocated second chromosome. The following strains originated spontaneously within these strains: ones with the translocated W chromosome, derived from the sex-limited + P (normal larval marking), sex-limited p (moricaud larval marking) (TAZIMAet al. 1955), sex-limited p B (black larval marking) (SASAKI1955), and other sex-limited strains (SASAKI1958). The chromosomes of B. mori are small, many (2n = 56), and not available to banding techniques, so that each individual chromosome has not yet been cytologically identified. Spermatocytes have been in general used for observing chromosomes because it is relatively easy to obtain chromosome images in dividing cells. Chromosomes at the pachytene stage from the oocyte are more suitable for observing chromosome morphology, even though it is very difficult to make good chromosome preparations in the female pachytene stage. Female pachytene chromosomes are larger, and do not overlap one another as much as the male ones do (RASMUSSEN1976; TRAUT 1976; KAWAMURAand NIINO 1991). Recently, KAWAMURA and NIINO (1991) and SAHARA(1998) found an asymmetrical bivalent while observing the + 96 N . Tanaka et al. pachytene chromosomes of oocytes in the sex-limited yellow blood strain. They have considered this bivalent to be the Z-W bivalent. A sex heterochromatin body (SB), deduced to be condensed W chromosomes (TRAUT and MosBACHER 1968; ENNIS 1976; TRAUT and MAREC 1996), has been observed in the highly polyploid interphase nuclei of Lepidopteran females. It has been reported that the number of SBs in a cell nucleus of B. mori corresponds closely to that of the W chromosomes in polyploids (ITO 1977; PAN et al. 1986, 1987). Conclusive evidence for the relationship between the SB and the W chromosome has been obtained with W chromosome mutant strains of Ephestiu kuehniella. Translocation or fusion of the W chromosome with an autosome or with the Z chromosome is accompanied by fragmentation of the SB in polyploid cells (TRAUT and RATHJENS1973; RATHJENS1974; TRAUTand SCHOLZ1978; TRAUT et al. 1986; MARECand TRAUT1994). The dispersed SBs in these cases were attributed to opposing tendencies during the polyploidization of the sticky heterochromatic segment (W chromosome) and adjacent non-sticky euchromatic chromosome segment (autosomes or Z chromosome) (TRAUTand RATHJENS 1973; RATHJENS 1974; MARECand TRAUT1994). We have found that the sex-limited p B silkworm strain (TWPB) has 27 chromosome pairs including an inordinately large chromosome pair in the pachytene stage of an oocyte, while a normal strain has 28 bivalents. In addition, we have obtained two new strains, sex-limited and autosomal p M-like strains (TWPML and T5PML strains, respectively), derived from the TWPB strain (unpublished). Here, we analyze the large chromosome by using the TWPB, TWPML, and T5PML strains. We have observed the female pachytene chromosomes and the SBs in the somatic cell nuclei and have made a linkage analysis using chromosomal marker genes. The results show the composition and the arrangement of the large chromosome of the TWPB strain and the chromosomal constitution of the TWPB-derived strains. Hereditas 133 (2000) allele of it. In the TWPB strain, the females have black larval marking, and the males have normal larval marking. Individuals of the N21 strain are homozygous for both pe (pink-eyed white egg, 5-0.0) and oc (Chinese translucent, 5-40.8). This strain was used as a marker for the fifth chromosome (linkage group) in the linkage analysis. Both pe and oc are recessive alleles. The linkage analysis was based on the circumstance that female silkworms do not have crossing over (TANAKAI91 3). Linkage analysis for the inordinately large chromosome of the TWPB strain was carried out between the W chromosome with the second chromosome fragment and the Z (sch), second (p Y), third ([em Z e ) , fifth (pe re oc), sixth (EKp),tenth (w2), 13th (ch), or 18th (rnln) chromosomes. When a dominant marker gene was used, the segregation in the F, was examined. When a recessive marker gene was used, the segregation in the BF, was examined. Pachytene chromosome preparations from oocytes were made by following TSUCHIDAet al. (1997). Ovaries were excised from fifth instar larvae on the third day and immersed in a hypotonic KCl solution (0.5 %) for 60 to 180 minutes. Oviducts were dissected from the ovaries with forceps in the hypotonic solution and, after washing, transferred to a 1.5 ml microtube with the KCl solution. The oviducts were slowly fixed with an MA solution (Carnoy’s fluid) (methanol 3: acetic acid I) and then squashed with a polytron homogenizer (KINEMATICA). The contents were dropped on slides in a humid box, and the slides were left overnight. The tissues were then stained with DAPI at room temperature for 10 min and observed under a Zeiss Axioskop microscope equipped with epifluorescent optics. The sex heterochromatin body (SB) was observed in the highly polyploid nuclei of sucking stomachs. In this material SB is easily detected in female individuals (PAN et al. 1987). Excised sucking stomachs were dissected on slides with forceps. The tissue was then stained by dropping acetic orcein (3 YO), covered with coverslips, pressed after 10 min, and observed by light microscopy. MATERIALS AND METHODS We have used the strains 5137, N21, TWPB, and the TWPB-derived strain, i.e., the sex-limited pM-like strain (TWPMLA, TWPML) and the autosomal p M like strain (TSPML). The TWPB strain, which is expected to be congenic to the 5137 strain ( +”/ +”) for the W chromosome, was constructed by backcrossing more than 15 times a female of the sex-limited p B strain from S A S A K(1955) ~ with a male of the 5137 strain (OHBAYASHIet al. 1996). + P is the normal larval marking gene, and p B is a dominant RESULTS The large chromosome of the sex-limited p silkworm strain (TWPB) In the pachytene chromosomes from oocytes, the 5137 strain of a chromosomally normal type had 28 bivalents (Fig. la), while the TWPB strain had 27 chromosome pairs (Fig. 1b) including one inordinately large chromosome pair (see arrowhead). The large chromosome pair was about 1.3 times longer Hereditas 133 (2000) than the second longest bivalent, and that shape was symmetrical and smooth. Since the TWPB strain used here was constructed by backcrossing a sex-limited p silkworm strain with a male of the J137 strain (n = 28), one would expect that the large chromosome of the TWPB strain would be formed by an attachment between the W chromosome with the second chromosome fragment (p” fragment) and another chromosome. We prepared sucking stomachs of the 5137 and the TWPB strains, and inspected in detail for the presence and shape of SBs in their highly polyploid nuclei. In the 5137 strain, a single SB was regularly observed in each nucleus of the female moths (Fig. 2a), while no SB was detected in nuclei of the male moths (Fig. 2b). On the other hand, in the TWPB strain, several SBs in each nucleus (four in F i g . 2 ~and Fusion chromosome in Bombyx mori 97 at least five in Fig.2d) were observed in the female moths, SBs were absent from the nuclei of the male moths. The proportion of nuclei displaying dispersed SBs in the TWPB females was clearly higher than that in the 5137 females. The SB in the TWPB strain was similar to that in W chromosome mutant strains of Ephestia kuehniella. These have a fused chromosome formed with the W chromosome and another chromosome. Evidently, the W chromosome with the second chromosome fragment of the TWPB strain is attached to an unknown chromosome. To determine the composition of the large chromosome, we crossed the females of the TWPB strain with males of strains having marker genes on various chromosomes, and examined the segregation in BF, and/or F, progeny. Consequently, in the BF, progeny obtained by crossing the female of the TWPB strain with the male of N21 strain homozygous for both pe and oc genes on the fifth chromosome, individuals hatched from normal pigmented eggs [ + ”‘1 were females with black larval marking [pq except for one individual, and individuals hatched from white eggs [pel were males with normal larval marking [ + P I except for two individuals (Table 1). Accordingly, it was evident that the large chromosome of the TWPB strain is composed of the W chromosome, the second chromosome fragment and the fifth chromosome. In addition, in the F, progeny obtained by crossing the females of the TWPB strain with the males homozygous for pe (5-0.0) and oc (5-40.8), or re (5-3 1.7), individuals for these three kinds of recessive genes failed to appear. This means that the fifth chromosome of the large chromosome in the TWPB y e , and + ‘IL genes. strain has + P e , + The strain derived from the exceptional individuals obtained for linkage analysis on the T W P B strain ( TW P M L A ) Fig. 1. Pachytene chromosomes of oocytes. a Normal strain (J137). Twenty-eight bivalents are observed. b Sexlimited p B silkworm strain (TWPB). Twenty-seven chromosome pairs are observed. Arrowhead indicates a large chromosome pair. Bar = 10 urn. In the TWPB strain there arise individuals with moricaud-like (p M-like) larval marking and detachment of the translocated second chromosome fragment from the W chromosome, though at very low frequency (unpublished). Therefore, we expected that the exceptional individuals (Table 1) would be like the pM-like males hatched from [ + which arose as a result of detachment of the W chromosome from the large chromosome, and p M-like females hatched from [pel arose as a result of detachment of the fifth chromosome from the large chromosome. As for the two females with p M-likelarval marking that hatched from the [pel, we wanted to confirm the expected detachment. We investigated the filial segregation of characteristics by crossing the above two females with seiae males of the N21 strain (Table 21. 98 N . Tanaka et al. Hereditas 133 (2000) Fig. 2. Highly polyploid nuclei of sucking stomach cells. a Female nucleus of 5137 strain. Arrowhead indicates an SB. b Male nucleus of 5137 strain. No SB is observed. c and d Female nuclei of TWPB strain. Arrowheads indicate dispersed SB fragments. Bar = 10 pm The results showed that all offspring from the two females had the [pel and the [oc], and the linkage between the second chromosome fragment and the W chromosome was maintained, except for six females with the [ + P I . Consequently, the above pM-like females arose as a result of the detachment of the fifth chromosome from the large chromosome. Among the female pachytene chromosomes derived from batch "b" in Table 2, 27 chromosome pairs including one large chromosome pair were observed (Fig. 3a). Furthermore, as for the SBs in each nucleus for these females, several SBs in each nucleus were found in the fashion of the TWPB strain (Fig. 3b). The above results suggested that the females with the sex-limited pM-like larval marking arose as a result of the detachment of the fifth chromosome from the large chromosome, and by the subsequent attachment with another chromosome. We attempted to select from the batches for sexlimited pM-like silkworms with [pel and [oc], and we called the batches, which had a stable expression of p M-like larval marking, the TWPMLA strain. We made a linkage analysis for the large chromosome of the TWPMLA strain. This analysis suggests that an unknown chromosome attached with the W chroniosome having the second chromosome fragment is not a Z chromosome. Table 1. Linkage analysis of the large chromosome of the TWPB strain for thejifth chromosome +P' 0 s pe +oc PB +p PB 224 0 2* 0 1* 0 * Body color shows pM-likemarking. OL +p 0 212 Fusion chromosome in Bombvx mori Hereditas 133 (2000) 99 Table 2. Segregation of larval marking and sex in the cross of the pM-likefemales in Table 1 x p e oc males p e oc p M like a b p s F like Q s 19 23 0 0 43 0 o +p 6 F S 10 21 0 0 4 47 53 31 0 6 100 2 Results of two batches and their sum are listed. Sex-limited p *-like silkworm strain derived from the T W P B strain ( T W P M L ) We subjected females of the sex-limited p M-like silkworm strain (TWPML) derived from the TWPB strain to linkage analysis between the W chromosome with the second chromosome fragment (p *-like fragment) and the fifth chromosome (Table 3). The results of [p*-like]:[ +"] = 1:1 and [Q]:[S] = 1:1 in individuals hatched from both [ +]'P and [ 9 show that the W chromosome with the second chromosome fragment is not linked with the fifth chromosome. Also, in the female pachytene chromosomes of the TWPML strain, 28 bivalents were observed as in the normal strain and unlike the TWPB strain (Fig. 4a). Furthermore, in the female cell nuclei from the sucking stomach of the TWPML strain, a single SB in each nucleus was in general observed (Fig. 4b). These results suggest that the TWPML strain has originated through the detachment of the fifth chromosome from the large chromosome of the TWPB strain. + Autosomal p M-likesilkworm strain derived from the T W P B strain ( T S P M L ) We subjected females of the autosomal p M-like silkworm strain (TSPML) derived from the TWPB strain to linkage analysis between the second chromosome fragment (p M-like fragment) and the fifth chromosome (Table 4). The results show that individuals hatched from the [ +""I had the p'-like larval marking, except for four individuals, and those hatched from the [pel had the p larval marking (except for four individuals). It is apparent that the second chromosome fragment is linked to the fifth chromosome. This means that in the large chromosome of the TWPB strain, the second chromosome fragment is attached to the fifth chromosome. Also, in the female pachytene chromosome of the T5PML strain, 28 bivalents were observed in the fashion of the normal strain (Fig. 5a). In the sucking stomach cells of the + Fig. 3. TWPMLA strain. a Pachytene chromosomes of an oocyte. Twenty seven chromosome pairs are observed. Arrowhead indicates a large chromosome pair. b Female nucleus of a sucking stomach cell with a string of SBs (arrowhead). Bar = 10 pm. female moths derived from the individuals with pM-like larval marking of the TSPML strain, a single SB in each nucleus was in general detected (Fig. 5b). In the nuclei of the male moths derived from the individuals with pM-like larval marking of the Table 3. Linkage analysis of the p'-like gene in the sex-limited pM-like strain ( T W P M L ) derived from the T W P B strain for the fifth chromosome p M-like ? s 195 0 +p pM-like 0 210 190 0 +" 0 166 100 N . Tanaka et al. Hereditas 133 (2000) strain is arranged in order W chromosome-second chromosome fragment-fifth chromosome. Furthermore, this fifth chromosome contains the loci from P" (0.0) to OC (40.8) at least. Also, since there is little difference in the growth rate between females with large chromosomes and males without large chromosomes, it is expected that the about complete fifth chromosome of the large chromosome is attached to the second chromosome fragment. Accordingly, we conclude that the large chromosome of the TWPB strain has originated from a reciprocal translocation with break points close to telomeres in both the second chromosome fragment linked with the W chromosome and the fifth chromosome. The chromosome constitutions of the chromosome mutant strains are illustrated as pachytene pairing configurations in female meiosis (Fig. 6). We made a crossing over experiment using the TSPML strain for the mapping of the pM-like gene (data not shown). It seems likely that the fifth chromosome of the large chromosome in the TWPB strain is attached to the second chromosome fragment close to the terminal site in + O" direction. Several cases of spontaneous chromosome fusion have been reported in B. mori. Examples include fusions between the sixth and 14th chromosomes (ITIKAWA1952; CHIKUSHI1959), the sixth and seventh (SAKAIDAet al. 1996), the 23rd and 25th (DOIRA et al. 1987; BANNOet al. 1993), the fifth (lacking of the +p' locus) and the normal W (ONIMARU and TAZIMA1983), so far. Because of preparation difficulties, no cases of chromosome fusions have been observed at the pachytene stage. The images of fused chromosomes shown here are the first instances at the female pachytene stage of B. mori. Body colors of mutant strains derived from the TWPB strain (TWPMLA, TWPML, and TSPML) changed from the p larval marking into the p M-like larval marking, and the origin of three strains was accompanied by partial detachment of the fused chromosome of the TWPB strain (see the Fig. 6). Though all such detached individuals do not display body coior variations, it is expected that the body color change from the p B larval marking into the pM-like larval marking shows a sign of detachment at the fused chromosome. We shall give a detailed evaluation of multiple expression of the larval marking due to the p locus of the translocated second chromosome fragment elsewhere. ITIKAWA(1952) irradiated individuals having attached sixth and 14th chromosomes and obtained 3.3 % detached individuals. This detachment was confirmed through genetic analysis. The primary spermatocytes of these individuals had either 26 or 27 chromosome pairs. ITIKAWA(1952) inferred that this + Fig. 4. TWPML strain. a Pachytene bivalents of an oocyte. Twenty-eight bivalents are observed. b Female nucleus of a sucking stomach cell with a normal SB (arrowhead). Bar = 10 ym. TSPML strain, SB was not detected (Fig. 5c). These results suggest that the TSPML strain was obtained by the detachment of the W chromosome from the large chromosome of the TWPB strain. DISCUSSION The results on the TWPB, TWPML, and T5PML strains show that the large chromosome of the TWPB Table 4. Linkage analysis of the pM-like gene in the autosovial p M-like strain (TSPML) derived from the TWPB strain for the fifth chromosome p M-like 5 8 I53 168 +p p M-like 2 2 2 2 +" 144 136 + Fusion chromosome in Bombyx mori Hereditas 133 (2000) 101 Fig. 5. TSPML strain. a Pachytene bivalents of an oocyte. Twenty-eight bivalents are observed. b Female nucleus of a sucking stomach cell with a normal SB (arrowhead). c Male nucleus of a sucking stomach cell without an SB. Bar = 10 pm. W -W A z A W (In +" +'" v +pB Fig. 6. Female pachytene configuration of normal and mutant strains in the present study. (a) Normal strain (5137). (b) W chromosome-pB fragment-fifth chromosome fusion strain (TWPB). (c) W chromosome-p ""-like fragment-autosome fusion strain (TWPMLA). (d) W chromosome-p M-like fragment translocation strain (TWPML). (e) fifth chromosome-p M-likefragment translocation strain (TSPML). W: W chromosome, Z: Z chromosome, V: fifth chromosome, A: Autosome, (11): second chromosome fragment, *: The arrangement of the three chromosomes (components) was assumed with n o confirmation. 102 N . Tunaka et ul. was due to the reattachment of the detached chromosome(s) for other chromosomes. Our cytogenetic analysis shows that the TWPMLA strain has arisen through a detachment of the fifth chromosome from the fused chromosome of the TWPB strain and the W chromosome has been subsequently attached to an unknown autosome. This is analogous to the observation of ITIKAWA (1952). We have found an instance of breakage and subsequent reattachment for the fusion chromosome of the TWPB strain (TANAKAet al. 2000). In a W chromosome-autosome fusion strain of E. kuehniellu, TRAUT(1986) reported that an attached autosome was replaced by a different, non-homologous one. In conclusion, once a fusion chromosome is formed in Lepidoptera, and even if this fusion chromosome is detached, it becomes easy to form one (or two) fusion chromosome(s) involving the two detached chromosomes. One might, at least hypothetically, produce a series of sex-limited strains involving a fusion of W with another chromosome. Such sex-limited strains with probably complementary chromosomal constitutions are expected to have rather few individuals with growth abnormalities such as retarded growth of females in comparison to sex-limited strains that have arisen through translocation of the chromosomal fragment on the W chromosome. Strains of this kind might be useful for the improvement of commercial silkworms. The TWPB strain used here originated from the sex-limited p B silkworm found by SASAKI(1955). An analysis of the W chromosomes in different sex-limited p B silkworm strains derived from the one described by SASAKI (1955) would indicate how the fusion chromosome of the TWPB strain had originated. REFERENCES Banno Y, Kawaguchi Y and Doira H, (1993). Cytogenetical analysis of chromosomal aberration due to translocation between the 23rd and 25th linkage groups in the silkworm, Bombyx mori. Hereditas 118: 259-263. Chikushi H, (1959). Contributions to genetics of Bombyx, with special reference to mechanisms of manifestation of characteristics. I. Sci. Bull. Fac. Agri. Kyusyu Univ. 17: 171-187. (In Japanese with English summary). Doira H, Nakayama M, Banno Y and Kawaguchi Y, (1987). Genetical analysis of the Naked-t mutant in Bombyx mori. J. Seric. Sci. Jpn. 56: 220-225. (In Japanese with English summary). Ennis TJ, (1976). Sex chromatin and chromosome numbers in Lepidoptera. Can. J. Genet. Cytol. 18: 119-130. Fujii H, Banno Y, Doira H, Kihara H and Kawaguchi Y, (1998). Genetical Stocks and Mutations of Bombyx mori: Important Genetic Resources (ed.: H FUJII). Silkworm Genetic Division, Institute of Genetic Resources, Faculty of Agriculture, Kyusyu University, Fukuoka, Japan, p. 1-54. Hereditas 133 (2000) Hasimoto H, (1933). Genetical studies on the tetraploid female in the silkworm. Bull. Imp. Seric. Exp. Sta. Jap. 8: 359-381. (In Japanese with English summary). Hasimoto H, (1948). Sex-limited zebra, an X-ray mutation in the silkworm. J. Seric. Sci. Jpn. 16: 62-64. (In Japanese). Itikawa N, (1952). Genetical and embryological studies on the E-multipule allele in the silkworm, Bombyx mori. Bull. Seric. Exp. Sta. Jap. 14: 23-91. (In Japanese with English summary). Ito S, (1977). Cytogenetical studies on the chromosomes of silkgland cells of the silkworm with special reference to the structure and behavior of the sex chromosomes. Jpn. J. Genet. 52: 327-340. Kawaguchi E, (1934). Chromosome behaviour in tetraploid female of the silkworm. J. Seric. Sci. Jpn. 5: 73-79. (In Japanese). Kawamura N and Niino T, (1991). Identification of the Z-W bivalent in the silkworm, Bombyx mori. Genetica 83: 121-123. Kimura K, Harada C and Aoki H, (1971). Studies on the W translocation of yellow blood gene in the silkworm (Bombyx mori). Jpn. J. Breed. 21: 199-203. (In Japanese with English summary). Marec F and Traut W, (1994). Sex chromosome pairing and sex chromatin bodies in W-Z translocation strains of Ephestia kuehniella (Lepidoptera). Genome 37: 426435. Ohbayashi F, Abe H, Simada T, Kawai S, Yokoyama T, Oshiki T and Kobayashi M, (1996). A common random amplified polymorphic DNA in the silkworm, Bombyx mori is shared by W chromosomes onto which the normal marking, Sable and Black genes are translocated respectively. J. Seric. Sci. Jpn. 65: 395-398. (In Japanese). Onimaru K and Tazima Y, (1983). On a W.V translocation strain of the silkworm with special reference to its chromosome constitution, pairing and segregation. J. Seric. Sci. Jpn. 52: 126-132. (In Japanese with English summary). Pan Q Z , Sugai E and Oshiki T, (1986). Sex-chromatin in cell nuclei of abdominal leg of the silkworm, Bombyx mori. J. Seric. Sci. Jpn. 55: 483-487. (In Japanese with English summary). Pan QZ, Sugai E and Oshiki T, (1987). Sex-chromatin observation in cell nuclei of tissues of the silkworm moth, Bombyx mori. J. Seric. Sci. Jpn. 56: 436-439. (In Japanese with English summary). Rasmussen SW, (1976). The meiotic prophase in Bombyx mori females Analyzed by three-dimensional reconstructions of synaptonemal complexes. Chromosoma 54: 245-293. Rathjens B, (1974). Zur Funktion des W-Chromatins bei Ephestia kuehniella (Lepidoptera). Chromosoma 47: 21 -44. Sahara K, (1998). Genetic and morphological features in the polyploid silkworms induced by low-temperature treatment. Memo. Fac. Agr. Hokkaido Univ. 21: 55-99. (In Japanese with English summary). Sakaida K, Banno Y , Nakamura T, Kawaguchi Y, Koga K and Doira H, (1996). Aberrant meiotic behavior of chromosomes due to reciprocal translocation in the ED" mutant of the silkworm, Bombyx mori. Hereditas 125: 25-29. Sasaki S, (1955). Genetical studies on the new sex-limited black marking of silkworm larvae. Jpn. J. Genet. 30: 185. (In Japanese). Hereditas 133 (2000) Sasaki S, (1958). Studies on the breeding of a new sex-limited normal marking silkworm. Jap. J. Breed.8: 185. (In Japanese). Takasaki T, (1952). Cytogenetics. In: Genetics of Silkworm (ed. Y Tanaka). Shokabo Press, Tokyo, p. 275-350. (In Japanese). Tanaka N, Yokoyama T, Irobe Y , Abe H, Ninagi 0 and Oshiki T, (2000). Breakage and subsequent return for W chromosome--pB fragment-fifth linkage group fusion chromosome in the sex-limited p B silkworm strain (TWPB), Bombyx mori. J. Seric. Sci. Jpn. 69: 327-330. (In Japanese). Tanaka Y, (1913). A study of Mendelian factors in the silkworm, Bombyx mori. J. Coll. Agric. Tohoku. Imp. Univ. 5: 115-148. Tanaka Y , (1916). Genetic studies in the silkworm. J. Coll. Agric. Sapporo 6: 1-33. Tanaka Y, (1939). Non-disjunction of the sex-chromosomes in the silkworm. Jpn. J. Genet. 15: 359-361. (In Japanese). Tazima Y, (1941). A simple method of sex discrimination by means of larval markings in Bombyx mori. J. Seric. Sci. Jpn. 12: 184-188. (In Japanese). Tazima Y, (1942). Cytogenetical improvement of autosexing method with use of larval marking (I). J. Seric. Sci. Jpn. 13: 81-95. (In Japanese). Tazima Y, (1943). Cytogenetical improvement of autosexing method with use of larval marking (I). J. Seric. Sci. Jpn. 14: 76-89. (In Japanese). Tazima Y, (1944). Studies on chromosome aberrations in the silkworm. 11. Translocation involving second and W-chromosomes. Bull. Seric. Exp. Sta. Jap. 12: 109181. (In Japanese). Fusion chromosome in Bombyx mori 103 Tazima Y, Harada C and Ohta N, (1951). On the sex discriminating method by colouring genes of silkworm eggs. I. Induction of translocation between the W and the tenth chromosomes. Jap. J. Breed. 1: 47-50. (In Japanese with English summary). Tazima Y, Kume T and Kamioka M, (1955). A new method of sex discrimination of silkworm larvae by a sex-limited moricaud marking. Reports of the Silk Science Research Institute. 5: 5-24. (In Japanese with English summary). Traut W, (1976). Pachytene mapping in the female silkworm, Bombyx mori L. (Lepidoptera). Chromosoma 58: 275-284. Traut W, (1986). A genetic linkage study of W-chromosome-autosome fusions, breakage, and kinetic organization of chromosomes in Ephestia (Lepidoptera). Genetica 69: 69-79. Traut W and Marec F, (1996). Sex chromatin in Lepidoptera. Quart. Rev. Biol. 71: 239-256. Traut W and Mosbacher GC, (1968). Geschlechtschromatin bei Lepidopteren. Chromosoma 25: 343-356. Traut W and Rathjens B, (1973). Das W-Chromosom von Ephestia kuehniella (Lepidoptera) und die Ableitung des Geschlechtschromatins. Chromosoma 41: 437-446. Traut W and Scholz D, (1978). Structure, replication and transcriptional activity of the sex-specific heterochromatin in a moth. Exp. Cell Res. 113: 47-53. Traut W, Weith A and Traut G, (1986). Structural mutants of the W chromosome in Ephestia (Insecta, Lepidoptera). Genetica 70: 69-79. Tsuchida K, Banno Y, Hashido K, (1997). Methods for fluorescence in situ hybridization using oocyte and spermatocyte chromosomes of Bombyx mori. J. Seric. Sci. Jpn. 66: 233-241. (In Japanese with English summary).