* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Material since exam 3
Double-slit experiment wikipedia , lookup
Tight binding wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Renormalization wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Elementary particle wikipedia , lookup
Electron configuration wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electron scattering wikipedia , lookup
Wave–particle duality wikipedia , lookup
Particle in a box wikipedia , lookup
Hydrogen atom wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
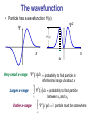
Material since exam 3 • De Broglie wavelength, wavefunctions, probabilities • Uncertainty principle • Particle in a box – Wavefunctions, energy, uncertainty relation – 1D, 2D, and 3D box, wavefunctions, energy • 3D hydrogen atom – Quantum #’s, physical meaning of quantum #’s – Energies and wavefunctions – Orbital magnetic dipole moment, electron spin • Multielectron atoms – State energies, electron configuration, periodic table – Lasers • Nuclear physics – Isotopes, nuclear binding energy – Radioactive decay • Decay rates, activity, radiation damage • Types of decay, half-life, radioactive dating. Matter waves • If light waves have particle-like properties, maybe matter has wave properties? • de Broglie postulated that the wavelength of matter is related to momentum as h p • This is called the de Broglie wavelength. Nobel prize, 1929 Matter Waves • deBroglie postulated that matter has wavelike properties. • deBroglie wavelength h / p Example: Wavelength of electron with 10 eV of energy: Kinetic energy p2 E KE p 2mE KE 2m h hc 1240eV nm 0.39nm 2 6 2mE KE 2mc E KE 20.51110 eV 10eV Heisenberg Uncertainty Principle • Using – x = position uncertainty – p = momentum uncertainty • Heisenberg showed that the product ( x ) ( p ) is always greater than ( h / 4 ) Often write this as x p ~ is pronounced ‘h-bar’ where h 2 /2 Planck’s constant The wavefunction • Particle has a wavefunction (x) 2 0.2 0.15 2(x) 0.1 0.05 0 x -0.05 -4 -3 -2 -1 0 1 2 dx Very small x-range x 3 4 2 xdx = probability to find particle in infinitesimal range dx about x x2 Larger x-range Entire x-range xdx = probability to find particle 2 x1 between x1 and x2 2 x dx 1 particle must be somewhere Question 2 0.5nm-1 0.2 0.15 0.1 What is probability that particle is found in 0.01nm wide region about -0.2nm? 0.05 0 A. 0.001 B. 0.005 C. 0.01 D. 0.05 E. 0.1 -0.05 -4 About what is probability that particle is in the region -1.0nm<x<0.0nm? A. 0.1 B. 0.4 C. 0.5 D. 1.5 E. 3.0 -0.8nm -3 -2 -0.2nm -1 0 0 1 2 x 3 Particle in 1D box h p2 E 2m h 2L /3 2 h 32 8mL2 n=2 h 2L /2 h2 2 8mL2 n=1 h 2L h2 8mL2 n n=3 p 2 L Wavefunction Probability • Quantized momentum h h p npo 2L /n • Energy = kinetic 2 2 npo p E n2Eo 2m 2m • Or Quantized Energy Energy Particle in box energy levels n=5 n=4 En n2Eo n=3 n=quantum number n=2 n=1 3-D particle in box: summary • Three quantum numbers (nx,ny,nz) label each state – nx,y,z=1, 2, 3 … (integers starting at 1) • Each state has different motion in x, y, z • Quantum numbers determine px – Momentum in each direction: e.g. h n nx x h 2L 2 2 p p p – Energy: E y z E o nx2 ny2 nz2 2m 2m 2m 2 x • Some quantum states have same energy Question How many 3-D particle in box spatial quantum states have energy E=18Eo? A. 1 B. 2 C. 3 D. 5 E. 6 E E o n x2 n y2 n z2 n ,n ,n 4,1,1, 1,4,1, (1,1,4) x y z Q: ask what state is this? (121) (211) All these states have the same energy, but different probabilities (112) 3D hydrogen atom mS Spin magnetic quantum number +1/2 or -1/2 2 For hydrogen atom: • • • • n describes energy of orbit : E 13.6 /n 2 eV ℓ describes the magnitude of orbital angular momentum m ℓ describes the angle of the orbital angular momentum of the spin angular moment ms describes the angle n 2 0 m 0 1 ms 2 Other elements: Li has 3 electrons n 2 0 m 0 1 ms 2 n 2 1 m 0 1 ms 2 n 2 1 m 0 1 ms 2 n 2 1 m 1 1 ms 2 n 2 1 m 1 1 ms 2 n 1 0 m 0 m s 1/2 n 2 1 m 1 1 ms 2 n=2 states, 8 total, 1 occupied n=1 states, 2 total, 2 occupied n 1 0 m 0 m s 1/2 n 2 1 m 1 1 ms 2 one spin up, one spin down Question Inert gas atoms are ones that have just enough electrons to finish filling a p-shell (except for He). How many electrons do next two inert gas atoms after helium ( neon (Ne) and argon (Ar) ) have. In this range of atomic number the subshells fill in order of increasing angular momentum. A. 10 & 18 B. 4 & 8 C. 8 & 16 D. 12 & 20 E. 6 & 10 Multi-electron atoms • Electrons interact with nucleus (like hydrogen) • Also with other electrons • Causes energy to depend on ℓ States fill in order of energy: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d Energy depends on n and ℓ Energy depends only on n The periodic table • Atoms in same column have ‘similar’ chemical properties. • Quantum mechanical explanation: similar ‘outer’ electron configurations. H 1s1 Li 2s1 Na 3s1 K 4s1 Be 2s2 Mg 3s2 Ca 4s2 Sc 3d1 Y 3d2 8 more transition metals B 2p1 Al 3p1 Ga 4p1 C 2p2 Si 3p2 Ge 4p2 N 2p3 P 3p3 As 4p3 O 2p4 S 3p4 Se 4p4 F 2p5 Cl 3p5 Br 4p5 He 1s2 Ne 2p6 Ar 3p6 Kr 4p6 Electron Configurations Atom Configuration H 1s1 He 1s2 Li 1s22s1 Be 1s22s2 B 1s22s22p1 Ne etc 1s shell filled 1s22s22p6 (n=1 shell filled noble gas) 2s shell filled 2p shell filled (n=2 shell filled noble gas) Ruby laser operation Relaxation to metastable state (no photon emission) 3 e V 2 Metastable state e V PUMP 1 e V Transition by stimulated emission of photon Ground state Isotopes # protons • Carbon has 6 protons, 6 electrons (Z=6): this is what makes it carbon. Total # nucleons 12 C 6 • Most common form of carbon has 6 neutrons in the nucleus. Called 12C Another form of carbon has 6 protons, 8 neutrons in the nucleus. This is 14C. This is a different ‘isotope’ of carbon Isotopes: same # protons, different # neutrons Nuclear matter Any particle in nucleus, neutron or proton, is called a nucleon. “A” is atomic mass number A=total number of nucleons in nucleus. Experimental result All nuclei have ~ same (incredibly high!) density of 2.3x1017kg/m3 Volume A = number of nucleons Radius A1/3 r ro A1/ 3, ro 1.2 fm 1.2 1015 m Binding energy • Calculate binding energy from masses E binding Zmp NmN mnucleusc 2 Zme Zme E binding ZmH NmN matom c 2 Mass of Hydrogen atom (1.0078 u) Mass of atom with Z protons, N neutrons Atomic masses well-known-> easier to use Biological effects of radiation • Radiation damage depends on – Energy deposited / tissue mass (1 Gy (gray) = 1J/kg) – Damaging effect of particle (RBE, relative biological effectiveness) Radiation type RBE X-rays Gamma rays Beta particles Alpha particles 1 1 1-2 10-20 • Dose equivalent = (Energy deposited / tissue mass) x RBE – Units of Sv (sieverts) [older unit = rem, 1 rem=0.01 Sv] – Common units mSv (10-3Sv), mrem (10-3rem) – Common ‘safe’ limit = 500 mrem/yr (5 mSv/yr) Exposure from 60Co source Co source has an activity of 1 µCurie 3.7 10 decays /s • 60 • Each decay: 1.3 MeV photon emitted • Hold in your fist for one hour 4 – all particles absorbed by a 1 kg section of your body for 1 hour • Energy absorbed in 1 kg = 1.310 eV 1.6 10 6 19 J /eV 3.7 104 decays /s1hr3600s/hr 2.8 105 J What dose do you receive? A. 0.5 rem 5 2.8 10 J /kg1rad /0.01J /kg B. 0.3 rem C. 0.1 rem D. 0.05 rem 0.003 rad = 0.003 rem E. 0.003 rem Quantifying radioactivity Decay rate r Prob( nucleus decays in time t ) = r t (Units of becquerel (1 Bq=1 s-1) or curie (1 Ci=3.7x1010 s-1) Activity R Mean # decays / s = rN, Half-life t1/2 (Units of s-1) N=# nuclei in sample (Units of s) time for half of nuclei to decay = t1/2 N N oer t ln 2 0.693 r r Activity of Radon • 222Rn has a half-life of 3.83 days. • Suppose your basement has 4.0 x 108 such nuclei in the air. What is the activity? We are trying to find number of decays/sec. So we have to know decay constant to get R=rN 0.693 0.693 r 2.09 106 s t1/ 2 3.83days 86,400s /day dN R rN 2.09 106 s 4.0 10 8 nuclei 836decays /s dt 1Ci R 836 decays /s 0.023Ci 10 2.7 10 decays /s Decay summary • Alpha decay – Nucleus emits He nucleus (2 protons, 2 neutrons) – Nucleus loses 2 protons, 2 neutrons • Beta- decay – Nucleus emits electron – Neutron changes to proton in nucleus • Beta+ decay – Nucleus emits positron – Proton changes to neutron in nucleus • Gamma decay – Nucleus emits photon as it drops from excited state