* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biehl PPT Part2
Survey
Document related concepts
Ring-closing metathesis wikipedia , lookup
Elias James Corey wikipedia , lookup
Bottromycin wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Sulfuric acid wikipedia , lookup
Asymmetric induction wikipedia , lookup
Discodermolide wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Transcript
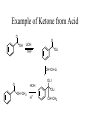
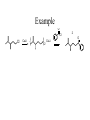
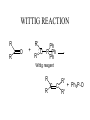
Synthesis of Ketones From Acids RCOOH + LiOH RCOO- Li+ + R’- Li+ Dry THF Dry RCOO- Li+ OLi R THF OLi R' DIANION IMPORTANT: NO WATER TO HERE OLi R R OLi R' OH HOH H+ O OH R' GEM DIOL R R' Example of Ketone from Acid O OH LiOH O OLi THF CH=CH-Li OLI O HOH CH=CH2 H+ OLi CH=CH2 Synthesis Using Acid and RLi Convert PhEt to Cyclopropyl ethyl ketone KMnO4 HOT 1. LiOH O OH How could one prepared benzoic acid From ethyl benzene? Side-chain oxidation!!!! Appears as if we could start with benzoic acid and introduce ethyl group by EtLi. 2. EtLi 3. HOH H+ O Et Synthesis of Aldehydes and Ketones from Acid Chlorides 1. Preparation of acid chlorides O O SOCl2 R OH R Cl R = almost anything!! H, Alkyl, Aryl, etc 2. Preparation of aldehydes - bulky reducing reagents O O AlH(Ot-But) 3 R Cl R Reduction to ROH does H not occur - steric Synthesis of Ketones Recall that ketones react with acid chlorides to give alcohols. O R O R'Li Cl R OH R'Li R' R' R R' OLi R Cl R' TO STOP AT KETONE STAGE, NEED A MILDER ORGANOLITHIUM USE OF ORGANO CUPRATES Rli + CuI --------> R2CuLi O R Cu + R R' O Cl R R' STOPS: addition to acid chloride is much faster than addition to ketone. Example O Cl Cl CuLi 2 CuLi 2 O Reactions of Carbonyls Nucleophilic addition - pages 797-800 Base-promoted additions Nucleophilic anion attacks C=O to give alkoxide which is neutralized to alcohol by acidic work-up. Acid-catalyzed additions O + +H + O + H Nu- O NuCOH H increases electrophilicity of carbonyl group Relative rate of addition: HCHO > RCHO> RCOR WITTIG REACTION R R O + R' Ph : C P Ph R' Ph Wittig reagent R R C C R' R' + Ph3P-O