* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 投影片 1
Survey
Document related concepts
Gene therapy wikipedia , lookup
Microevolution wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Gene nomenclature wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Nicotinic acid adenine dinucleotide phosphate wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Designer baby wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Transcript
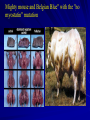
Gene Therapy in Improving Muscle Mass and Strength Effects of Follistatin and Myostatin on Muscle Growth Follistatin also known as activin-binding protein is a protein that in humans is encoded by the FST gene an autocrine glycoprotein that is expressed in nearly all tissues of higher animals a naturally occurring protein that inhibits myostatin, a growth factor expressed specifically in skeletal muscle. FST gene the promotion of cellular differentiation of the estrogen producing granulosa cells (GC) of the dominant follicle into the progesterone producing large lutein cells (LLC) of the corpus luteum. Structure Structure of the follistatin/activin complex FS Isoforms Three reported isoforms, FS-288, FS-300, and FS-315 FS-288 and FS-315, are known to be created by alternative splicing of the primary mRNA transcript FS-300 (porcine follistatin) is thought to be the product of posttranslational modification via truncation of the C-terminal domain from the primary amino-acid chain FS Isoforms FS is ubiquitous its highest concentration has been found to be in the female ovary, followed by the skin The activin-binding protein follistatin is produced by folliculostellate (FS) cells of the anterior pituitary FS cells make numerous contacts with the classical endocrine cells of the anterior pituitary including gonadotrophs FS Isoforms In the tissues activin has a strong role in cellular proliferation, and follistatin the controlled cellular proliferation and allowing it to function as an instrument of cellular differentiation In the blood, activin and follistatin are both known to be involved in the inflammatory response following tissue injury or pathogenic incursion FS Production The source of follistatin in circulating blood plasma has yet to be determined, but due to its autocrine nature speculation suggests the endothelial cells lining all blood vessels, or the macrophages and monocytes also circulating within the whole blood, may be sources. Functions Follistatin is being studied for its role in regulation of muscle growth in mice, as an antagonist to myostatin (also known as GDF-8, a TGF superfamily member) which inhibits excessive muscle growth. Functions Lee & McPherron demonstrated that inhibition of GDF-8, either by genetic elimination (knockout mice) or by increasing the amount of follistatin, resulted in greatly increased muscle mass In 2009, research with Macaque monkeys demonstrated that regulating follistatin via gene therapy also resulted in muscle growth and increases in strength Myostatin also known as growth differentiation factor 8 (GDF-8) is a secreted TGF beta protein family member that inhibits muscle differentiation and growth. Myostatin is produced primarily in skeletal muscle cells, circulates in the blood and acts on muscle tissue, by binding a cell-bound receptor called the activin type II receptor Myostatin In humans, myostatin is encoded by the MSTN gene. The myostatin gene is expressed almost exclusively in cells of skeletal-muscle lineage throughout embryonic development as well as in adult animals and functions as a negative regulator of muscle growth Myostatin Animals lacking myostatin or animals treated with substances such as follistatin that block the binding of myostatin to its receptor have significantly larger muscles MSTN gene Mighty mouse and Belgian Blue" with the "no myostatin" mutation Functions Myostatin is active in muscles used for movement (skeletal muscles) both before and after birth. This protein normally restrains muscle growth, ensuring that muscles do not grow too large Functions Mutations that reduce the production of functional myostatin lead to an overgrowth of muscle tissue Myostatin-related muscle hypertrophy has a pattern of inheritance known as incomplete autosomal dominance Functions People with a mutation in both copies of the MSTN gene in each cell (homozygotes) have significantly increased muscle mass and strength. People with a mutation in one copy of the MSTN gene in each cell (heterozygotes) also have increased muscle bulk, but to a lesser degree Functions A German boy was diagnosed with a mutation in both copies of the myostatin-producing gene, making him considerably stronger than his peers. His mother, a former sprinter, has a mutation in one copy of the gene An American boy defect in his myostatin receptor makes his muscles not respond to the myostatin signal Biochemistry/Physiology Myostatin is a member of the TGF beta superfamily of proteins. Human myostatin consists of two identical subunits, each consisting of 109 amino acid residues Biochemistry/Physiology Its total molecular weight is 25.0 kDa. The protein is made in an inactive form For it to be activated, a protease cleaves the NH3-terminal, or "pro-domain" portion of the molecule, resulting in the now-active COOHterminal dimer Biochemistry/Physiology Myostatin binds to the activin type II receptor, resulting in a recruitment of a coreceptor called Alk-3 or Alk-4 (a transducer of activin or activin like ligands (e.g., inhibin) signals This coreceptor then initiates a cell signaling cascade in the muscle, which includes the activation of transcription factors in the SMAD family - SMAD2 and SMAD3. Biochemistry/Physiology These factors then induce myostatin-specific gene regulation. When applied to myoblasts, myostatin inhibits their differentiation into mature muscle fibers Myostatin has also been shown to inhibit Akt, a kinase which is sufficient to cause muscle hypertrophy, in part through the activation of protein synthesis. Biochemistry/Physiology Therefore myostatin acts in two ways, by inhibiting muscle differentiation, and by inhibiting Akt-induced protein synthesis Recent study showed that a two-week treatment of normal mice with soluble activin type IIB receptor, a molecule that is normally attached to cells and binds to myostatin, leads to a significantly increased muscle mass (up to 60%) Biochemistry/Physiology It is thought that binding of myostatin to the soluble activin receptor prevents it from interacting with the cell-bound receptors Exercise Inhibits Myostatin RNA Expression Fold changes in myostatin mRNA expression following an acute bout of RE or RUN normalized to GAPDH mRNA and relative to preexercise levels. Total RNA was extracted from the muscle biopsies of the vastus lateralis (RE) or gastrocnemius (RUN). Values are means ± SE. *P < 0.05 from preexercise mRNA expression. Louis E et al. J Appl Physiol 2007;103:1744-1751 It remains unclear whether long term treatment of muscular dystrophy with myostatin inhibitors is beneficial Perspective in Sports Science Discussion For the purpose to promote muscle function, can you use the myostain’s knowledge to treat the people having weak muscle. In your opinion, an adequate strength training would produce a muscle hypertrophy may be a result of follistatin stimulation or myostain inhibition or both. Considering exercises performance, why athletes do not use follistain to improve their muscle strength, what is the reason?