* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch. 6 - Department of Chemistry and Biochemistry
Cracking (chemistry) wikipedia , lookup
Kinetic isotope effect wikipedia , lookup
Discodermolide wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Elias James Corey wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Petasis reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Stille reaction wikipedia , lookup
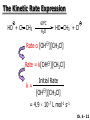
Physical organic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Marcus theory wikipedia , lookup
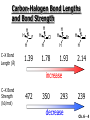
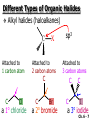
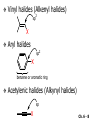
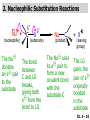
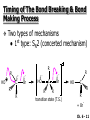
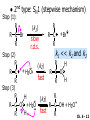
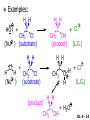
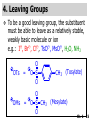
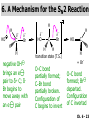
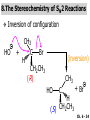
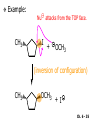
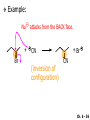
Chapter 6 Nucleophilic Substitution and Elimination Reactions of Alkyl Halides Created by Professor William Tam & Dr. Phillis Chang Ch. 6 - 1 About The Authors These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in research and teaching, and according to Essential Science Indicators, he is currently ranked as the Top 1% most cited Chemists worldwide. He has published four books and over 80 scientific papers in top international journals such as J. Am. Chem. Soc., Angew. Chem., Org. Lett., and J. Org. Chem. Dr. Phillis Chang received her B.Sc. at New York University (USA) in 1994, her M.Sc. and Ph.D. in 1997 and 2001 at the University of Guelph (Canada). She lives in Guelph with her husband, William, and their son, Matthew. Ch. 6 - 2 1. Organic Halides C X X = Cl, Br, I Halogens are more electronegative than carbon Ch. 6 - 3 Carbon-Halogen Bond Lengths and Bond Strength H H C F H C–X Bond Length (Å) 1.39 H H H C Br H H H H C I H 1.78 1.93 2.14 C Cl H increase C–X Bond Strength (kJ/mol) 472 350 293 decrease 239 Ch. 6 - 4 1A. Physical Properties of Organic Halides: Boiling Point (bp/oC) Group Fluoride Chloride Bromide Iodide Me -78.4 -23.8 3.6 42.5 Et -37.7 13.1 38.4 72 Bu 32 78.4 101 130 s Bu - 68 91.2 120 i Bu - 69 91 119 12 51 73.3 100(dec) t Bu Ch. 6 - 5 Physical Properties of Organic Halides: Density (r) Group Fluoride Chloride Bromide Iodide Me 0.84(-60) 0.92 1.73(0) 2.28 Et 0.72 0.91(15) 1.46 1.95 Bu 0.78 0.89 1.27 1.61 s Bu - 0.87 1.26 1.60 i Bu - 0.87 1.26 1.60 0.75(12) 0.84 1.22 1.57(0) t Bu Ch. 6 - 6 Different Types of Organic Halides Alkyl halides (haloalkanes) C Attached to 1 carbon atom X Attached to 2 carbon atoms sp3 Attached to 3 carbon atoms C C Cl a 1o chloride C C Br a 2o bromide C C I a 3o iodide Ch. 6 - 7 Vinyl halides (Alkenyl halides) sp2 X Aryl halides sp2 X benzene or aromatic ring Acetylenic halides (Alkynyl halides) sp X Ch. 6 - 8 sp C X 3 Alkyl halides sp2 X Prone to undergo Nucleophilic Substitutions (SN) and Elimination Reactions (E) (the focus of this Chapter) sp2 X sp X Different reactivity than alkyl halides, and do not undergo SN or E reactions Ch. 6 - 9 2. Nucleophilic Substitution Reactions Nu + (nucleophile) The Nu⊖ donates an e⊖ pair to the substrate C X (substrate) The bond between C and LG breaks, giving both e⊖ from the bond to LG Nu C (product) The Nu⊖ uses its e⊖ pair to form a new covalent bond with the substrate C + X (leaving group) The LG gains the pair of e⊖ originally bonded in the substrate Ch. 6 - 10 Timing of The Bond Breaking & Bond Making Process Two types of mechanisms ● 1st type: SN2 (concerted mechanism) R HO R C R R Br R HO Br C R R transition state (T.S.) HO C R R + BrCh. 6 - 11 ● 2nd type: SN1 (stepwise mechanism) Step (1): R R C Br R (k 1 ) slow r.d.s. R R C R + Br k1 << k2 and k3 Step (2) R R H (k 2 ) R C + H 2O R C O fast H R R Step (3) R R H (k3) + + + R C O H 2O R C OH H3O fast H R R Ch. 6 - 12 3. Nucleophiles A reagent that seeks a positive center Has an unshared pair of e⊖ e.g.: HO , CH3O , H2N (negative charge) H2O, NH3 (neutral) This is the positive center that the Nu⊖ seeks C X Ch. 6 - 13 Examples: H H HO + C CH3 Cl (Nu ) (substrate) H H + O C H H CH3 Cl (Nu ) (substrate) H H + Cl C CH3 OH (product) (L.G.) H H C H + Cl CH3 O (L.G.) H H H (product) C + H3O CH3 OH Ch. 6 - 14 4. Leaving Groups To be a good leaving group, the substituent must be able to leave as a relatively stable, weakly basic molecule or ion e.g.: I⊖, Br⊖, Cl⊖, TsO⊖, MsO⊖, H2O, NH3 OTs = O O S O OMs = O O S CH3 (Mesylate) O CH3 (Tosylate) Ch. 6 - 15 5. Kinetics of a Nucleophilic Substitution Reaction: An SN2 Reaction HO + CH3 Br HO CH3 + Br - Rate = k[CH3Br][OH ] The rate of the substitution reaction is linearly dependent on the concentration of OH⊖ and CH3Br Overall, a second-order reaction bimolecular Ch. 6 - 16 5A. How Do We Measure the Rate of This Reaction? e.g.: HO (Nu ) H + H H C Cl H (substrate) HO C H H (product) + Cl (leaving group) The rate of reaction can be measured by ● The consumption of the reactants (HO⊖ or CH3Cl) or ● The appearance of the products (CH3OH or Cl⊖) over time Ch. 6 - 17 Concentration, M Graphically… [CH3Cl] ↓ [CH3OH] ↑ Time, t Rate = Δ[CH3Cl] Δt =− [CH3Cl]t=t − [CH3Cl]t=0 Time in seconds Ch. 6 - 18 Concentration, M Initial Rate [CH3Cl]t=0 [CH3Cl]t=t [CH3Cl] Time, t [CH3Cl]t=t − [CH3Cl]t=0 Initial Rate =− (from slope) Δt Ch. 6 - 19 Example: HO + Cl CH3 [OH⊖]t=0 60oC H2O [CH3Cl]t=0 HO CH3 + Cl Initial rate mole L-1, s-1 Result 1.0 M 0.0010 M 4.9 × 10-7 1.0 M 0.0020 M 9.8 × 10-7 Doubled 2.0 M 0.0010 M 9.8 × 10-7 Doubled 2.0 M 0.0020 M 19.6 × 10-7 Quadrupled Ch. 6 - 20 Conclusion: HO + Cl CH3 60oC H2O HO CH3 + Cl ● The rate of reaction is directly proportional to the concentration of either reactant. ● When the concentration of either reactant is doubled, the rate of reaction doubles. Ch. 6 - 21 The Kinetic Rate Expression HO + Cl CH3 60oC H2O HO CH3 + Cl Rate α [OH⊖][CH3Cl] Rate = k[OH⊖][CH3Cl] k= Initial Rate [OH⊖][CH3Cl] = 4.9 × 10-7 L mol-1 s-1 Ch. 6 - 22 6. A Mechanism for the SN2 Reaction H HO H C Br H H negative OH⊖ brings an e⊖ pair to δ+ C; δ– Br begins to move away with an e⊖ pair H HO Br C H H transition state (T.S.) O–C bond partially formed; C–Br bond partially broken. Configuration of C begins to invert HO C H H + Br- O–C bond formed; Br⊖ departed. Configuration of C inverted Ch. 6 - 23 7. Transition State Theory: Free Energy Diagrams A reaction that proceeds with a negative free-energy change (releases energy to its surroundings) is said to be exergonic A reaction that proceeds with a positive free-energy change (absorbs energy from its surroundings) is said to be endergonic Ch. 6 - 24 At 60oC (333 K) CH3 Br + OH CH3 OH + Cl DGo = -100 kJ/mol ● This reaction is highly exergonic DHo = -75 kJ/mol ● This reaction is exothermic Ch. 6 - 25 CH3 Br + OH CH3 OH + Cl ● Its equilibrium constant (Keq) is ln Keq = = DGo = –RT ln Keq –DGo RT –(–100 kJ/mol) (0.00831 kJ K-1 mol-1)(333 K) = 36.1 Keq = 5.0 ╳ 1015 Ch. 6 - 26 A Free Energy Diagram for a Hypothetical SN2 Reaction That Takes Place with a Negative DGo Ch. 6 - 27 The reaction coordinate indicates the progress of the reaction, in terms of the conversion of reactants to products The top of the energy curve corresponds to the transition state for the reaction The free energy of activation (DG‡) for the reaction is the difference in energy between the reactants and the transition state The free energy change for the reaction (DGo) is the difference in energy between the reactants and the products Ch. 6 - 28 A Free Energy Diagram for a Hypothetical Reaction with a Positive Free-Energy Change Ch. 6 - 29 7A. Temperature, Reaction Rate, and the Equilibrium Constant Distribution of energies at two different temperatures. The number of collisions with energies greater than the free energy of activation is indicated by the corresponding shaded area under each curve. A 10°C increase in temperature will cause the reaction rate to double for many reactions taking place near room temperature Ch. 6 - 30 The relationship between the rate constant (k) and DG‡ is exponential : k = k0 e DG‡/RT e = 2.718, the base of natural logarithms k0 = absolute rate Distribution of energies at two different temperatures. The number of collisions with energies greater than the free energy of activation is indicated by the corresponding shaded area under each curve. constant, which equals the rate at which all transition states proceed to products (At 25oC, k0 = 6.2 ╳ 1012 s1 ) Ch. 6 - 31 Distribution of energies at two different temperatures. The number of collisions with energies greater than the free energy of activation is indicated by the corresponding shaded area under each curve. A reaction with a lower free energy of activation (DG‡) will occur exponentially faster than a reaction with a higher DG‡, as dictated by k = k0 e DG‡/RT Ch. 6 - 32 Free Energy Diagram of SN2 Reactions Free Energy T.S. DG HO- + CH3Br DGo DG = free energy of activation DGo = free energy change CH3OH + Br- Reaction Coordinate Exothermic (DGo is negative) Thermodynamically favorable process Ch. 6 - 33 8.The Stereochemistry of SN2 Reactions Inversion of configuration HO CH3 + C Br H CH2CH3 (R) (inversion) CH3 HO C H (S) CH2CH3 + Br Ch. 6 - 34 Example: CH3 Nu⊖ attacks from the TOP face. I + OCH3 (inversion of configuration) CH3 OCH3 + I Ch. 6 - 35 Example: Nu⊖ attacks from the BACK face. + Br + Br CN (inversion of configuration) CN Ch. 6 - 36 9. The Reaction of tert-Butyl Chloride with Hydroxide Ion: An SN1 Reaction CH3 CH3 C Br + H2O CH3 CH3 CH3 C OH + HBr CH3 The rate of SN1 reactions depends only on concentration of the alkyl halide and is independent on concentration of the Nu⊖ Rate = k[RX] In other words, it is a first-order reaction unimolecular nucleophilic substitution Ch. 6 - 37 9A. Multistep Reactions & the RateDetermining Step In a multistep reaction, the rate of the overall reaction is the same as the rate of the SLOWEST step, known as the rate-determining step (r.d.s) For example: Reactant k1 k2 Intermediate (slow) (fast) 1 k3 Intermediate (fast) 2 k1 << k2 or k3 Product Ch. 6 - 38 A B C The opening A is much smaller than openings B and C The overall rate at which sand reaches to the bottom of the hourglass is limited by the rate at which sand falls through opening A Opening A is analogous to the rate-determining step of a multistep Ch. 6 - 39 reaction 10. A Mechanism for the SN1 Reaction A multistep process Step (1): CH3 CH3 C Br CH3 (k 1 ) slow r.d. step CH3 CH3 C + Br CH3 (ionization of alkyl halide) Ch. 6 - 40 Free Energy Free Energy Diagram of SN1 Reactions T.S. (1) T.S. (2) T.S. (3) (CH3)3C + BrDG1 (CH3)3CBr + H2O (CH3)3C -OH2 + Br- intermediate (CH3)3C-OH + Br- Reaction Coordinate Ch. 6 - 41 Step (2) CH3 CH3 C + H2O CH3 (k 2 ) fast CH3 H CH3 C O CH3 H Ch. 6 - 42 Free Energy Free Energy Diagram of SN1 Reactions T.S. (1) T.S. (2) T.S. (3) (CH3)3C - + Br DG1 (CH3)3C -OH2 - + Br (CH3)3CBr + H2O intermediate (CH3)3C-OH - + Br Reaction Coordinate Ch. 6 - 43 Step (2) CH3 CH3 C + H2O CH3 (k2) fast CH3 H CH3 C O CH3 H Step (3) CH3 H CH3 C O + H2O CH3 H (k 3 ) fast CH3 CH3 C OH CH3 + + H3OCh. 6 - 44 Free Energy Free Energy Diagram of SN1 Reactions T.S. (1) T.S. (2) T.S. (3) (CH3)3C - + Br DG1 (CH3)3C -OH2 - + Br (CH3)3CBr + H2O intermediate (CH3)3C-OH - + Br Reaction Coordinate Ch. 6 - 45 Step (2) CH3 CH3 C + H2O CH3 (k2) fast Step (3) CH3 H CH3 C O + H2O CH3 H CH3 H CH3 C O CH3 H k1 << k2 and k3 (k3) fast CH3 CH3 C OH CH3 + H3O+ Ch. 6 - 46 2 intermediates and 3 transition states (T.S.) The most important T.S. for SN1 reactions is T.S. (1) of the ratedetermining step (r.d.s.) CH3 CH3 C Br CH3 Ch. 6 - 47 11. Carbocations 11A. The Structure of Carbocations Carbocations are trigonal planar The central carbon atom in a carbocation is electron deficient; it has only six e⊖ in its valence shell The p orbital of a carbocation contains no electrons, but it can accept an electron pair when the carbocation undergoes further reaction H3C H3C C CH3 sp2-sp3 p bond Ch. 6 - 48 11B. The Relative Stabilities of Carbocations General order of reactivity (towards SN1 reaction) ● 3o > 2o >> 1o > methyl The more stable the carbocation formed, the faster the SN1 reaction Ch. 6 - 49 Stability of cations most stable (positive inductive effect) R R C R > R R C R > H H C H > H H C H Resonance stabilization of allylic and benzylic cations CH2 CH2 etc. Ch. 6 - 50 12. The Stereochemistry of SN1 Reactions Ph CH3 Ph CH3OH Br CH2CH3 (S ) C CH3 CH2CH3 Ph CH3 CH2CH3 (R) CH3 C OCH3 CH2CH3 (R) and (S) racemic mixture (trigonal planar) CH3OH attack from left CH3O CH3OH Ph CH3OH attack from right 50:50 chance Ph CH3 (1 : 1) OCH3 CH2CH3 (S ) Ch. 6 - 51 racemic mixture ( 1 : 1 ) Example: (R) Br H2O (SN1) (R) (carbocation) OH H2O attack from TOP face H2O + OH (one enantiomer) slow r.d.s. (S) H2O H O H2O attack from H BOTTOM face H O H Ch. 6 - 52 Example: I t Me OMe Me MeOH tBu Bu Me +tBu OMe MeOH slow r.d.s. Me t MeOH Bu trigonal planar MeOH Me Me CH3 t Bu O H t MeOH Bu O Me H Ch. 6 - 53 13. Factors Affecting the Rates of SN1 and SN2 Reactions The structure of the substrate The concentration and reactivity of the nucleophile (for SN2 reactions only) The effect of the solvent The nature of the leaving group Ch. 6 - 54 13A. The Effect of the Structure of the Substrate General order of reactivity (towards SN2 reaction) ● Methyl > 1o > 2o >> 3o > vinyl or aryl DO NOT undergo SN2 reactions Ch. 6 - 55 For example: R Br + HO R OH + Br Relative Rate (towards SN2) CH3 CH3CH2 Br CH3CH Br CH3 C CH2Br CH3 CH3 CH3 Br methyl 2 10 6 Most reactive 1 o 4 10 2 4 o 500 CH3 CH3 C Br CH3 o neopentyl 3 1 <1 Least reactive Ch. 6 - 56 Compare H HO C H H H HO C CH3 CH3 H Br faster HO C H H Br H + Br slower HO + Br CH3 CH3 C Ch. 6 - 57 H HO C t Bu HO C CH3 Br H CH3 CH3 H very slow C HO CH3 + Br C CH3 CH3 Br + Br t Bu CH3 HO extremely slow Ch. 6 - 58 Note NO SN2 reaction on sp2 or sp carbons e.g. sp2 H I No reaction + Nu H H sp2 I + Nu No reaction sp I + Nu No reaction Ch. 6 - 59 Reactivity of the Substrate in SN1 Reactions General order of reactivity (towards SN1 reaction) ● 3o > 2o >> 1o > methyl The more stable the carbocation formed, the faster the SN1 reaction Ch. 6 - 60 Stability of cations most stable (positive inductive effect) R R C R > R R C R > H H C H > H H C H Allylic halides and benzylic halides also undergo SN1 reactions at reasonable I rates Br an allylic bromide a benzylic iodide Ch. 6 - 61 Resonance stabilization for allylic and benzylic cations CH2 CH2 etc. Ch. 6 - 62 13B. The Effect of the Concentration & Strength of the Nucleophile For SN1 reaction Recall: Rate = k[RX] ● The Nu⊖ does NOT participate in the r.d.s. ● Rate of SN1 reactions are NOT affected by either the concentration or the identity of the Nu⊖ Ch. 6 - 63 For SN2 reaction Recall: Rate = k[RX][RX] ● The rate of SN2 reactions depends on both the concentration and the identity of the attacking Nu⊖ Ch. 6 - 64 Identity of the Nu⊖ ● The relative strength of a Nu⊖ (its nucleophilicity) is measured in terms of the relative rate of its SN2 reaction with a given substrate rapid CH3O + CH3I CH3OCH3 + I Good Nu⊖ Very CH3OH + CH3I slow CH3OCH3 + I Poor Nu⊖ Ch. 6 - 65 The relative strength of a Nu⊖ can be correlated with 3 structural features ● A negatively charged Nu⊖ is always a more reactive Nu⊖ than its conjugated acid e.g. HO⊖ is a better Nu⊖ than H2O and RO⊖ is better than ROH ● In a group of Nu⊖s in which the nucleophilic atom is the same, nucleophilicities parallel basicities e.g. for O compounds, RO⊖ > HO⊖ >> RCO2⊖ > ROH > H2O Ch. 6 - 66 ● When the nucleophilic atoms are different, then nucleophilicities may not parallel basicities e.g. in protic solvents HS⊖, CN⊖, and I⊖ are all weaker bases than HO⊖, yet they are stronger Nu⊖s than HO⊖ HS⊖ > CN⊖ > I⊖ > HO⊖ Ch. 6 - 67 13C. Solvent Effects on SN2 Reactions: Polar Protic & Aprotic Solvents Classification of solvents Non-polar solvents (e.g. hexane, benzene) Solvents Polar solvents Polar protic solvents (e.g. H2O, MeOH) Polar aprotic solvents (e.g. DMSO, HMPA) Ch. 6 - 68 SN2 Reactions in Polar Aprotic Solvents ● The best solvents for SN2 reactions are Polar aprotic solvents, which have strong dipoles but do not have OH or NH groups Examples CH3 O S O H CH3 (DMSO) CH3 N CH3 (DMF) O P NMe Me2N NMe2 2 (HMPA) CH3CN (Acetonitrile) Ch. 6 - 69 Polar aprotic solvents tend to solvate metal cations rather than nucleophilic anions, and this results in “naked” anions of the Nu⊖ and makes the e⊖ pair of the Nu⊖ more available CH3O Na DMSO CH3O + DMSO Na "naked anion" Ch. 6 - 70 Tremendous acceleration in SN2 reactions with polar aprotic solvent CH3Br + NaI CH3I + NaBr Solvent Relative Rate MeOH 1 DMF 106 Ch. 6 - 71 SN2 Reactions in Polar Protic Solvents ● In polar protic solvents, the Nu⊖ anion is solvated by the surrounding protic solvent which makes the e⊖ pair of the Nu⊖ less available and thus less reactive in SN2 reactions H OR RO H Nu H OR H OR Ch. 6 - 72 Halide Nucleophilicity in Protic Solvents ● I⊖ > Br⊖ > Cl⊖ > F⊖ OR RO H RO H H - H F H OR H RO H OR I- H RO OR (strongly solvated) H OR (weakly solvated) Thus, I⊖ is a stronger Nu⊖ in protic solvents, as its e⊖ pair is more available to attack the substrate in the SN2 reaction. Ch. 6 - 73 Halide Nucleophilicity in Polar Aprotic Solvents (e.g. in DMSO) ● F⊖ > Cl⊖ > Br⊖ > I⊖ Polar aprotic solvents do not solvate anions but solvate the cations The “naked” anions act as the Nu⊖ Since F⊖ is smaller in size and the charge per surface area is larger than I⊖, the nucleophilicity of F⊖ in this environment is greater than I⊖ Ch. 6 - 74 13D. Solvent Effects on SN1 Reactions: The Ionizing Ability of the Solvent Solvent plays an important role in SN1 reactions but the reasons are different from those in SN2 reactions Solvent effects in SN1 reactions are due largely to stabilization or destabilization of the transition state Ch. 6 - 75 Polar protic solvents stabilize the development of the polar transition state and thus accelerate this ratedetermining step (r.d.s.): CH3 - CH3 C Cl CH3 Cl + CH3 slow r.d.s. CH2 C CH3 H3C CH3 C CH3 R O H OR H Cl H OR Ch. 6 - 76 13E. The Nature of the Leaving Group The better a species can stabilize a negative charge, the better the LG in an SN2 reaction SN1 Reaction: C X C slow r.d.s. SN2 Reaction: Nu: slow C X r.d.s. Nu C X C + X X Nu C +X Ch. 6 - 77 Examples of the reactivity of some X⊖: CH3O + CH3–X CH3–OCH3 + X Relative Rate: ⊖ Best X ⊖ OH, Worst X NH2, <<F < Cl < Br < I < TsO RO ~0 1 200 10,000 30,000 60,000 Note: Normally R–F, R–OH, R–NH2, R–OR’ do not undergo SN2 reactions. Ch. 6 - 78 Nu R OH R Nu + a poor leaving group OH a strong basic anion H R O H Nu ✔ a good H leaving group R Nu + H2O weak base Ch. 6 - 79 14. Organic Synthesis: Functional Group Transformation Using SN2 Reactions OH CN CN HO Me Br MeS SMe MeO HS SH Ch. 6 - 80 Me O I I Me C C Br N3 N3 O Me NMe3 Br MeCOO Me3N Ch. 6 - 81 Examples: NaOEt,??DMSO Br I O NaSMe,?? DMSO SMe Ch. 6 - 82 Examples: ?? I CN (optically active, chiral) (optically active, chiral) ● Need SN2 reactions to control stereochemistry ● But SN2 reactions give the inversion of configurations, so how do you get the “retention” of configuration here?? ● Solution: “double inversion” “retention” Ch. 6 - 83 ?? I CN (optically active, chiral) (optically active, chiral) NaBr DMSO NaCN DMSO (SN2 with inversion) Br (SN2 with inversion) (Note: Br⊖ is a stronger Nu than I⊖ in polar aprotic solvent.) Ch. 6 - 84 14A. The Unreactivity of Vinylic and Phenyl Halides X C C X vinylic halide phenyl halide Vinylic and phenyl halides are generally unreactive in SN1 or SN2 reactions Ch. 6 - 85 Examples NaCN Br DMSO I NaSMe HMPA No Reaction No Reaction Ch. 6 - 86 15. Elimination Reactions of Alkyl Halides Substitution H C C Br OCH3 (acts as a Nu ) H C C OCH3 + Br - Elimination H OCH3 C C (acts as a C C + CH3OH + Br H Br base) Ch. 6 - 87 Substitution reaction (SN) and elimination reaction (E) are processes in competition with each other e.g. I t BuOK t BuOH t O Bu + SN2: 15% E2: 85% Ch. 6 - 88 15A. Dehydrohalogenation β hydrogen β carbon Br β α H LG H C α carbon C X halide as LG t BuOK t BuOH, 60oC + KBr t + BuOH β hydrogen ⊖OtBu Ch. 6 - 89 15B. Bases Used in Dehydrohalogenation Conjugate base of alcohols is often used as the base in dehydrohalogenations Na R−O−H e.g. EtO Na NaH R−O⊖ + Na⊕ + H2 R−O⊖ + Na⊕ + H2 t BuO K sodium ethoxide potassium tert-butoxide Ch. 6 - 90 16. The E2 Reaction Br EtO + + EtOH + Br H Rate = k[CH3CHBrCH3][EtO⊖] Rate determining step involves both the alkyl halide and the alkoxide anion A bimolecular reaction Ch. 6 - 91 Mechanism for an E2 Reaction Et O CH3 α Cβ C H H Br H H EtO⊖ removes a b proton; C−H breaks; new p bond forms and Br begins to depart Et O H H C CH3 C H H Br Partial bonds in the transition state: C−H and C−Br bonds break, new p C−C bond forms H H C C + Et OH CH3 H + Br C=C is fully formed and the other products are EtOH and Br⊖ Ch. 6 - 92 Free Energy Diagram of E2 Reaction Free Energy T.S. DG‡ E2 reaction has ONE transition state CH3CHBrCH3 - + EtO CH2=CHCH3 + EtOH + Br - Reaction Coordinate Rate = k[CH3CHBrCH3][EtO⊖] Second-order overall bimolecular Ch. 6 - 93 17. The E1 Reaction E1: Unimolecular elimination CH3 CH3 CH 3 H 2O CH3 C OH + CH2 C CH3 C Cl CH3 CH3 CH3 (major (SN1)) (minor (E1)) slow r.d.s H O as H O as 2 2 CH3 base nucleophile CH C 3 CH3 Ch. 6 - 94 Mechanism of an E1 Reaction α carbon β hydrogen Cl H2O H H2O slow r.d.s. fast + H3O (E1 product) fast H2O O H HO 2 H OH + H3O (SN1 product) Ch. 6 - 95 Free Energy Free Energy Diagram of E1 Reaction T.S. (1) T.S. (2) (CH3)3C DG1 (CH3)3CCl + Cl(CH3)2C=CH2 + H3O + Cl- + H2O Reaction Coordinate Ch. 6 - 96 Step (1): CH3 CH3 C Cl CH3 Aided by the polar solvent, a chlorine departs with the e⊖ pair that bonded it to the carbon H 2O (k 1 ) slow r.d. step CH3 CH3 C + Cl CH3 Produces relatively stable 3o carbocation and a Cl⊖. The ions are solvated (and stabilized) by surrounding H2O molecules Ch. 6 - 97 Free Energy Free Energy Diagram of E1 Reaction T.S. (1) T.S. (2) (CH3)3C - DG1 + Cl (CH3)2C=CH2 - (CH3)3CCl + H3O + Cl + H2O Reaction Coordinate Ch. 6 - 98 Step (2) H 3C H 3C H C C H + H 2O (k 2 ) H 3C fast H 3C H H2O molecule removes one of the b hydrogens which are acidic due to the adjacent positive charge. An e⊖ pair moves in to form a double bond between the b and a carbon atoms CH2 + H O H H Produces alkene and hydronium ion Ch. 6 - 99 18. How To Determine Whether Substitution or Elimination Is Favoured All nucleophiles are potential bases and all bases are potential nucleophiles Substitution reactions are always in competition with elimination reactions Different factors can affect which type of reaction is favoured Ch. 6 - 100 18A. SN2 vs. E2 (b) Nu (a) H C C X (a) H C S N2 Nu C (b) C E2 C +X + Nu H + X Ch. 6 - 101 Primary Substrate With a strong base, e.g. EtO⊖ ● Favor SN2 NaOEt Br EtOH OEt SN2: 90% + E2: (10%) Ch. 6 - 102 Secondary Substrate With a strong base, e.g. EtO⊖ ● Favor E2 + Br NaOEt EtOH E2: 80% + OEt SN2: 20% Ch. 6 - 103 Tertiary Substrate With a strong base, e.g. EtO⊖ ● E2 is highly favored Br NaOEt EtOH + E2: 91% OEt SN1: 9% Ch. 6 - 104 Base/Nu⊖: Small vs. Bulky Unhindered “small” base/Nu⊖ NaOMe Br MeOH + OMe SN2: 99% E2: 1% Hindered “bulky” base/Nu⊖ t KO Bu Br t BuOH t + O Bu SN2: 15% E2: 85% Ch. 6 - 105 Basicity vs. Polarizability O O O CH3 CH3 C O (weak base) Br EtO (strong base) + SN2: 100% E2: 0% OEt + SN2: 20% E2: 80% Ch. 6 - 106 Tertiary Halides: SN1 vs. E1 & E2 Br EtO OEt + (strong base) E2: 100% SN1: 0% EtOH OEt + heat E1 + E2: 20% SN1: 80% Ch. 6 - 107 19. Overall Summary CH3X RCH2X R' RCHX R' RCX R" SN1 SN2 E1 E2 ─ Very fast ─ ─ ─ Mostly Very little; Mostly SN2 with Solvolysis possible; weak bases; e.g. with H2O; e.g. with CH3COO⊖ MeOH Very favorable with weak bases; e.g. with H2O; MeOH ─ ─ Hindered bases give mostly alkenes; e.g. with tBuO⊖ Very little Strong bases promote E2; e.g. with RO⊖, HO⊖ Strong bases Always competes promote E2; with SN1 e.g. with RO⊖, HO⊖ Ch. 6 - 108 Review Problems (1) t Bu Br Na CN CN DMF, 25oC SN2 with inversion t Bu (2) I O H O NaH Et2O H⊖ I O Intramolecular SN2 Ch. 6 - 109 (3) t CH3 OH CH3 Cl HCl t Bu Bu Cl + CH3 t Bu ( 50 : 50) Cl⊖ attacks from top face t Bu CH3 H O H SN1 with racemization CH3 t Bu Cl⊖ attacks from bottom face sp2 hybridized carbocation Ch. 6 - 110 END OF CHAPTER 6 Ch. 6 - 111