* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Autonomy Trial - Diabetes In Control
Survey
Document related concepts
Transcript
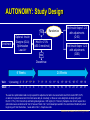
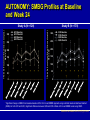
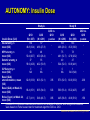
AUTONOMY: The First Randomized Trial Comparing Two Patient-driven Approaches to Initiate and Titrate Prandial Insulin Lispro in Type 2 Diabetes Steve V. Edelman,1Rong Liu,2 Jennal Johnson,2 Leonard C. Glass2 Edelman et al. Diabetes Care 2014;37(8):2132-40. This information may contain a substantive discussion of developmental compound(s) not approved for marketing in the United States and/or products approved for marketing in the United States but for uses, dosages, formulations and/or populations different than those discussed in these materials. If the information contains a substantive discussion of a currently marketed product(s), a URL address to the U.S. Prescribing Information for that product(s) is provided immediately below. You should consult this prescribing information for the product’s approved uses and important information, including boxed warnings, regarding the product’s use. Humalog (insulin lispro injection, USP [rDNA origin]) Product Information http://pi.lilly.com/us/humalog-pen-pi.pdf AUTONOMY: Introduction (Slide 1 of 2) Management of patients with type 2 diabetes generally requires stepwise intensification of therapy1,2: • Lifestyle changes, OADs, and noninsulin injectable antidiabetic agents • Given the progressive deterioration in β-cell function, progressing to addition of exogenous insulin Results of the United Kingdom Prospective Diabetes Study (UKPDS) support the need for treatment intensification with exogenous insulin in combination with OADs in a significant percentage of patients to achieve and maintain metabolic control3 1. Inzucchi et al. Diabetes Care 2012;35(6):1364-79(Updated 36: p 490). 2. Garber et al. Endocr Pract 2013;19(2):327-36. 3. Turner et al. JAMA 1999;281(21):2005-12. AUTONOMY: Introduction (Slide 2 of 2) Given limited data and myriad treatment approaches, there is currently no global clinical consensus for the approach to treatment intensification with insulin therapy Meta-analysis indicates most effective use of insulin is achieved using a basal/bolus regimen1 Currently, there is great interest in sequential addition of prandial insulin in patients with type 2 diabetes not attaining adequate glycemic control with basal insulin only2,3 1. Giugliano et al. Diabetes Care 2011;34(2):510-7. 2. Rodbard et al. Lancet Diabetes Endocrinol 2014;2(1):30-7. 3. Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Study Objective To compare efficacy and safety of 2 patient-based self-titration algorithms for initiation and titration of prandial insulin lispro in patients with type 2 diabetes inadequately controlled on basal insulin plus OADs in endocrine and generalist settings • Approximately 44% of trial sites were in primary care settings 1st comparison of 2 self-titration insulin algorithms for escalation of prandial insulin lispro therapy in a large, multicountry, randomized, controlled trial • Designed as 2 independent parallel studies (Study A, N = 528; Study B, N = 578) utilizing a single protocol to corroborate substantial evidence of reproducibility; data for each study were analyzed separately and independently Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Study Design NO Enrollment Optional: Insulin Glargine (GLA) Optimization Lead-ina Add insulin lispro 1-2-3 with adjustments (Q1D) Randomize HbA1c ≤7.0% (≤53.0 mmol/mol) Add insulin lispro 1-2-3 with adjustments (Q3D) YES Discontinue 6 Weeks Visit: 24 Weeks 1 (screening) 2a 3a 4a,b 5a,b 6a Week: -1 a6-week 0 1 2 4 6 7a 8b 9b 10 11b 12 13b 14b 15 16b 17 18b 19 7 8 9 10 12 16 23 29 14 18 19 27 31 GLA optimization lead-in only required for subjects who had to be converted to GLA from insulin NPH, ILPS, or detemir; required conversion from GLA twice daily to once daily; or those on once-daily GLA at study entry with HbA1c >7.0% (>53.0 mmol/mol) and fasting blood glucose >120 mg/dL (>6.7 mmol/L). Subjects who did not require GLA optimization were randomized at Visit 2, forewent Visits 3 to 7 and instead proceeded to the randomized treatment period beginning with Visit 8 activities, 1 week after Visit 2; bTelephone visits Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Treatment Algorithms Randomize 1:1 Q1D Q3D Self-titrated daily based on premeal glucose readings from prior day (eg, prebreakfast dose adjusted using prior day prelunch reading) Self-titrated every 3 days based on median blood glucose readings from the 3 days prior (eg, prebreakfast dose adjusted using median prelunch blood glucose reading from the past 3 days) Premeal Target Blood Glucose: 85-114 mg/dL Blood Glucose Median Reading: Blood Glucose Daily Reading: 85-114 mg/dL No change in dose 85-114 mg/dL No change in dose Target not achieved Increase dose by 1 U/day until target is reached 115-144 mg/dL Dose increased by 2U ≥145 mg/dL Dose increased by 4U 56-84 mg/dL Dose decreased by 1U 56-84 mg/dL Dose decreased by 2U <56 mg/dL Dose decreased by 2U <56 mg/dL Dose decreased by 4U Starting insulin lispro dose: 10% of total insulin glargine dose; first dose administered with breakfast unless subject does not eat breakfast, then first dose administered at lunch time at investigator discretion Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Inclusion Criteria Type 2 diabetes 18-85 years of age BMI <45 kg/m2 HbA1c >7.0% and ≤12.0% (>53.0 and ≤107.7 mmol/mol) Treated with ≥20 U/d of insulin glargine (GLA), neutral protamine Hagedorn (NPH), insulin lispro protamine suspension (ILPS), or detemir; and had been using metformin, meglitinide, sulfonylurea, pioglitazone, sitagliptin, or a combination of these for ≥3 months • Meglitinides or sulfonylureas were discontinued at randomization Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Exclusion Criteria Prior rapid- or short-acting insulin therapy Excessive insulin resistance (>2 U/kg/day) Morbid obesity (BMI ≥45 kg/m2) Pregnancy or planned pregnancy Cancer Recent history of severe hypoglycemia Moderate to severe cardiovascular, renal, hepatic, or hematologic disease Treatment with GLP-1 receptor agonists, α-glucosidase inhibitors, DPP-4 inhibitors (except sitagliptin), and rosiglitazone within 3 months, or glucocorticoids within 2 weeks of screening Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Primary and Secondary Outcome Measures Primary efficacy outcome: • Compare change in HbA1c from baseline to endpoint (Week 24 after randomization) for Q1D and Q3D algorithms Secondary outcomes: • Incidence and annualized rate of self-reported total, severe, and nocturnal hypoglycemia • Proportion of subjects achieving target values HbA1c ≤7.0% • Change in FBG, 7-point SMBG profile, and weight from baseline • Change in dose of basal (GLA) and prandial (lispro) insulin at end of study • Change in 1,5-anhydroglucitol (a marker of hyperglycemia) Assessments in subjects ≥65 years of age: • Change in HbA1c, hypoglycemia (incidence and rate), FBG, and proportion of subjects achieving target Safety was monitored throughout the study (hypoglycemia was considered an AE with severe hypoglycemia recorded as an SAE) Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Statistical Analyses (Slide 1 of 3) A classification method was applied for primary efficacy analysis, as no prior projection, preference, or historical evidence regarding which self-titration algorithm performs better was available1 All safety outcomes were assessed in the entire randomized population (ie, all subjects who entered the study, completed the GLA optimization lead-in period [if applicable], and were randomized) All efficacy analyses were based upon the full analysis set (subjects in the all randomized population who took at least 1 dose of insulin lispro), and a sensitivity analysis was conducted for the primary efficacy measure based upon the all-completer population All efficacy and safety analyses were conducted at an α-level of .05 All CIs were computed as 2-tailed using a 95% significance level For categorical measures, including AEs and hypoglycemia incidence, Fisher’s exact test or Pearson’s chi-square test was used 1. Qu et al. Stat Med 2011;30(30):3488-95. Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Statistical Analyses (Slide 2 of 3) Continuous efficacy and safety variables measured repeatedly were evaluated using a mixed model repeated measure (MMRM) approach using the restricted maximum likelihood method, including the following independent variables: • Fixed effects for treatment algorithm, all stratification variables, visit, treatment-by-visit interaction, and baseline outcome variable as the covariate Treatment-by-age group (≥65 years, <65 years) interaction for the change in HbA1c was tested using another MMRM model with additional items, including: • Subgroup and subgroup-by-treatment algorithm interaction Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Statistical Analyses (Slide 3 of 3) Hypoglycemia incidence was analyzed with a logistic model with terms for treatment algorithm and all stratification variables as sensitivity analysis The rate of total, nocturnal, and severe hypoglycemia per year during the treatment phase was analyzed using LOCF, applying a negative binomial model with terms for treatment algorithm, HbA1c stratum, sulfonylurea/meglitinide use, and the logarithm of the exposure time (in days) as an offset variable and compound symmetry as variancecovariance structure A Wilcoxon rank-sum test was conducted as a sensitivity analysis The percentages of subjects achieving HbA1c targets at the end of the study (LOCF) were analyzed using a logistic regression model with terms for treatment algorithm and strata All data are expressed as least square mean ± standard error unless otherwise stated, and a p-value of <.05 was considered significant Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Patient Disposition of All Randomized Subjects Study B Randomized (N = 581) Study A Randomized (N = 531) Q1D (N = 268)a Q3D (N = 263)a Q1D (N = 289)a Completed, n = 223 (83.2%) Completed, n = 210 (79.8%) Early terminated, n = 45 (16.8%) Early terminated, n = 53 (20.2%) Completed, n = 244 (84.4%) Early terminated, n = 45 (15.6%) Q3D (N = 292)a Completed, n = 241 (82.5%) Early terminated, n = 51 (17.5%) Reasons: n % Reasons: n % Reasons: n % Reasons: n % Adverse event 1 0.4 Adverse event 4 1.5 Adverse event 2 0.7 Adverse event 3 1.0 Death 2 0.7 Death 0 0.0 Death 1 0.3 Death 3 1.0 Entry criteria not met 1 0.4 Entry criteria not met 3 1.1 Entry criteria not met 4 1.4 Entry criteria not met 7 2.4 Lack of efficacy 1 0.4 Lack of efficacy 2 0.8 Lack of efficacy 1 0.3 Lack of efficacy 0 0.0 Lost to follow-up 4 1.5 Lost to follow-up 4 1.5 Lost to follow-up 8 2.8 Lost to follow-up 9 3.1 Physician decision 4 1.5 Physician decision 10 3.8 Physician decision 8 2.8 Physician decision 11 3.8 13 4.9 Protocol violation 8 2.8 Protocol violation 8 2.7 Protocol violation 17 6.3 Protocol violation Sponsor decision 1 0.4 Sponsor decision 2 0.8 Sponsor decision 0 0.0 Sponsor decision 1 0.3 Subject decision 14 5.2 Subject decision 15 5.7 Subject decision 13 4.5 Subject decision 9 3.1 a6 subjects were randomized but did not receive at least 1 dose of lispro: Study A Q1D (n = 1) and Q3D (n = 2); Study B Q1D (n = 1) and Q3D (n = 2). These subjects (ie, subjects not exposed to lispro) were not included in the full analysis set. Patient disposition was based on all randomized subjects. There was no significant difference between the percentages of subjects who discontinued from Q1D or Q3D for any reason of early termination. Deaths were not attributed to the treatment Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Baseline Demographics Baseline Demographics Age, years, mean (SD) Subjects ≥65 years, % Caucasian, % Female, % BMI, kg/m2, mean (SD) BMI ≥30kg/m2, % Body weight, kg (SD) Duration of diabetes, years, mean (SD) Duration >10 years HbA1c %, mean (SD) Study A Study B Q1D Q3D Q3D vs. Q1D Q1D Q3D Q3D vs. Q1D (N = 267) (N = 261) p-value (N = 288) (N = 290) p-value 57.9 (10.3) 58.8 (9.5) .278 57.7 (9.7) 57.0 (10.6) .412 24.3 26.4 .618 19.4 22.4 .414 82.3 83.5 .781 79.7 83.3 .308 49.8 52.9 .487 53.8 53.4 .934 33.3 (5.3) 33.4 (5.5) .793 32.6 (5.2) 33.2 (5.7) .174 73.4 69.7 .385 66.3 68.6 .594 94.6 (20.2) 92.4 (17.7) .188 90.8 (18.3) 93.5 (21.2) .112 11.7 (6.3) 12.6 (7.9) .129 11.6 (6.5) 11.9 (7.1) .645 54.3 8.3 (0.9) 60.2 8.4 (1.0) .188 .453 54.5 8.3 (1.0) 53.8 8.4 (1.0) .868 .162 mmol/mol, mean (SD) 67.2 (9.8) 68.3 (10.9) - HbA1c >8.0% (>63.93 mmol/mol), % 56.6 58.2 .725 67.2 (10.9) 68.3 (10.9) 53.5 57.6 .357 p-values for continuous measures were based on an analysis of variance, and categorical measures were based on Fisher’s exact test for treatment algorithm Q3D vs. Q1D Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Concomitant Medications Study A Study B Q1D (N = 267) Q3D (N = 261) Q1D (N = 288) Q3D (N = 290) Biguanides, % 85.4 89.3 93.8 89.3 Sulfonylurea/meglitinide, % 49.4 52.5 34.7 40.3 DPP-4 inhibitors, % 9.7 10.0 8.0 7.2 Thiazolidinediones, % 5.2 7.3 3.8 6.6 OAD class ≥2, % 44.9 51.0 36.1 39.3 Concomitant Medications p-values were not calculated Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Change from Baseline in HbA1c Study A: HbA1c (%) Study B: HbA1c (%) 0.0 Q1D Q3D N = 528 -0.2 -0.4 -0.6 * -0.8 * -1.0 * -1.2 Baseline Week 7 Week 12 Week 24 Q3D-Q1D LSM: 0.07 0.08 95% CI: -0.04, 0.17 -0.06, 0.22 0.04 -0.15, 0.22 HbA1c Change from Baseline (%, LSM SE) HbA1c Change from Baseline (%, LSM SE) 0.0 Q1D Q3D N = 578 -0.2 -0.4 -0.6 * -0.8 * -1.0 * -1.2 Baseline Week 7 Week 12 Week 24 Q3D-Q1D LSM: -0.01 -0.02 95% CI: -0.11, 0.09 -0.15, 0.11 0.06 -0.12, 0.24 *Significant change from baseline based on 95% CIs from an MMRM approach using restricted maximum likelihood method (REML) for both Q1D and Q3D Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Overall Percentage of Subjects with HbA1c ≤7.0% at 24 Weeks Study A (N = 528) Percentage of Subjects with HbA1c ≤7.0% at 24 Weeks 90% 100% p = .128 90% 80% 70% 60% 50% 49.8% 42.5% 40% 30% 20% Percentage of Subjects with HbA1c ≤7.0% at 24 Weeks 100% Study B (N = 578) 80% 70% 60% 50% 42.4% 30% 20% 10% 0% 0% Q3D 49.3% 40% 10% Q1D p = .162 Q1D Q3D No statistically significant difference between Q1D and Q3D algorithms for Study A and Study B for overall percentage of subjects reaching target HbA1c ≤7.0% (≤53.0 mmol/mol) at end of study (24 weeks) Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Subjects ≥65 Years of Age with HbA1c ≤7.0% at 24 Weeks Study A (N = 134)1 Study B (N = 121)1 100% p = .701 90% 80% 70% 60% 58.5% 58.0% 50% 40% 30% 20% Percentage of Subjects with HbA1c ≤7.0% at 24 Weeks Percentage of Subjects with HbA1c ≤7.0% at 24 Weeks 90% 100% *p = .015 80% 70% 67.9% 60% * 50% 46.2% 40% 30% 20% 10% 10% 0% 0% Q1D Q3D Q1D Q3D (n = 65)1 (n = 69)1 (n = 56)1 (n = 65)1 In Study B, percentage of subjects ≥65 years of age reaching target HbA1c ≤7.0% (≤53.0 mmol/mol) at end of study (24 weeks) was significantly lower for those randomized to Q3D (46.2%) than to Q1D (67.9%), p = .015; in Study A, there was no statistical difference between Q1 vs. Q3 algorithms Edelman et al. Diabetes Care 2014;37(8):2132-40. 1. Data on file, Eli Lilly and Company. AUTONOMY: SMBG Profiles at Baseline and Week 24 300 Study B (N = 578) Q1D Baseline Q1D Week 24 Q3D Baseline Q3D Week 24 250 200 150 100 50 0 * * * * * * 7-Point Self-monitored Blood Glucose (mg/dL) Mean SD 7-Point Self-monitored Blood Glucose (mg/dL) Mean SD Study A (N = 528) 300 250 Q1D Baseline Q1D Week 24 Q3D Baseline Q3D Week 24 200 150 100 50 * * † * * * * † † 0 *Significant change in SMBG from baseline based on 95% CIs from an MMRM approach using restricted maximum likelihood method (REML) for both Q1D and Q3D; †Significant difference between Q3D and Q1D at Week 24 from an MMRM model using REML Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Change from Baseline in 1,5-AG Study A: 1,5-AG (μg/mL) Study B: 1,5-AG (μg/mL) 3.0 4.0 Q1D Q3D N = 528 * 2.0 * 1.0 * 0.0 1,5-AG Change from Baseline (μg/mL, LSM SE) 1,5-AG Change from Baseline (μg/mL, LSM SE) 4.0 3.0 Q1D Q3D N = 578 * 2.0 * 1.0 * 0.0 Baseline Week 7 Week 12 Week 24 Q3D-Q1D LSM: -0.08 -0.29 95% CI: -0.54, 0.38 -0.88, 0.30 -0.15 -0.95, 0.66 Baseline Week 7 Week 12 Week 24 Q3D-Q1D LSM: 0.10 0.09 95% CI: -0.33, 0.53 -0.51, 0.70 -0.28 -1.08, 0.52 *Significant change from baseline based on 95% CIs from an MMRM approach using restricted maximum likelihood method (REML) for both Q1D and Q3D Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Overall Hypoglycemia Incidences and Rates per 1 Year Hypoglycemia Total: Q1D (N = 268) Study A Q3D vs. Q3D/Q1D Q3D Q1D Rate Ratio (N = 263) p-value (95% CI) Q1D (N = 289) Study B Q3D vs. Q3D/Q1D Q3D Q1D Rate Ratio (N = 292) p-value (95% CI) Incidence, n (%) 231 (86.2) 218 (83.2) .435 - 238 (82.4) 231 (79.1) .351 - Rate per 1 year,NBM (SE) 38.32 (2.80) 40.58 (3.06) .586 1.06 (0.86-1.30) 38.76 (3.14) 40.54 (3.29) .689 1.05 (0.84-1.30) Incidence, n (%) 194 (72.4) 191 (72.9) .923a .842b - 205 (70.9) 185 (63.4) .053a .032b - Rate per 1 year,NBM (SE) 20.88 (1.93) 24.16 (2.30) .272c .504d 1.16 (0.89-1.50) 18.22 (1.79) 21.26 (2.11) .260c .561d 1.17 (0.89-1.53) Incidence, n (%) 169 (63.1) 167 (63.7) .870 - 156 (54.0) 149 (51.0) .470 - Rate per 1 year,NBM (SE) 8.59 (0.80) 9.60 (0.93) .404 1.12 (0.86-1.45) 7.14 (0.80) 8.23 (0.91) .358 1.15 (0.85-1.56) Documented symptomatic1: Nocturnal: Incidence is reported as the number of subjects with at least 1 hypoglycemic episode; p-values for the incidences of each category were based on a logistic regression model for Q3D vs. Q1D; p-values for rate adjusted per 1 year were based on NBM regression for Q3D vs. Q1D; documented symptomatic p-values calculated by aFisher exact test, bLogistic regression model, cNegative binomial regression model, and dWilcoxon rank sum test 1. Data on file, Eli Lilly and Company. Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Overall Severe Hypoglycemia Incidences and Rates per 1 Year Study A Q3D vs. Q1D p-value Study B Q3D/Q1D Rate Ratio Q1D (95% CI) (N = 289) Q3D/Q1D Rate Ratio (95% CI) Q1D (N = 268) Q3D (N = 263) Incidence, n (%) 5 (1.9) 2 (0.8) .258 - 7 (2.4) 8 (2.7) .856 - Rate per 1 year, mean (SD) 0.04 (0.31) 0.03 (0.41) .271 - 0.11 (1.09) 0.06 (0.36) .816 - Hypoglycemia Severe: Q3D (N = 292) Q3D vs. Q1D p-value Incidence is reported as the number of subjects with at least 1 hypoglycemic episode; p-values for incidences were based on a logistic regression model for Q3D vs. Q1D; due to low occurrence of severe hypoglycemia, mean ± SD and only Wilcoxon test p-values are presented Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Hypoglycemia Incidences and Rates per 1 Year for Subjects ≥65 Years of Age Hypoglycemia Total: Q1D (N = 66) Study A Q3D vs. Q3D/Q1D Q3D Q1D Rate Ratio (N = 69) p-value (95% CI) Q1D (N = 56) Study B Q3D vs. Q3D/Q1D Q3D Q1D Rate Ratio (N = 65) p-value (95% CI) Incidence, n (%) 60 (90.9) 61 (88.4) .802 - 51 (91.1) 53 (81.5) .205 - Rate per 1 year,NBM (SE) 41.62 (5.42) 48.84 (6.21) .383 1.17 (0.82-1.68) 51.38 (8.26) 42.88 (6.35) .404 0.83 (0.55-1.28) Incidence, n (%) 52 (78.8) 56 (81.2) .831a .617b - 39 (69.6) 41 (63.1) .564a .265b - Rate per 1 year,NBM (SE) 20.84 (3.51) 28.30 (4.63) .197c .546d 1.36 (0.85-2.16) 22.48 (5.13) 19.06 (4.05) .593c .373d 0.85 (0.46-1.55) Incidence, n (%) 45 (68.2) 49 (71.0) .763 - 42 (75.0) 43 (66.2) .383 - Rate per 1 year,NBM (SE) 8.71 (1.48) 11.60 (1.92) .229 1.33 (0.84-2.12) 12.01 (2.40) 10.69 (2.03) .671 0.89 (0.52-1.52) Documented symptomatic1: Nocturnal: Incidence is reported as the number of subjects with at least 1 hypoglycemic episode; p-values for the incidences of each category were based on a logistic regression model for Q3D vs. Q1D; p-values for rate adjusted per 1 year were based on NBM regression for Q3D vs. Q1D; documented symptomatic p-values calculated by aFisher exact test, bLogistic regression model, cNegative binomial regression model, and dWilcoxon rank sum test 1. Data on file, Eli Lilly and Company. Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Severe Hypoglycemia Incidences and Rates per 1 Year for Subjects ≥65 Years of Age Study A Q3D vs. Q1D p-value Q3D/Q1D Rate Ratio (95% CI) Q1D (N = 66) Q3D (N = 69) Incidence, n (%) 3 (4.5) 1 (1.4) .296 Rate per 1 year, mean (SD) 0.10 (0.49) 0.03 (0.25) .294 Hypoglycemia Severe: Study B Q3D vs. Q1D p-value Q3D/Q1D Rate Ratio (95% CI) Q1D (N = 56) Q3D (N = 65) - 1 (1.8) 2 (3.1) .797 - - 0.05 (0.37) 0.07 (0.38) .657 - Incidence is reported as the number of subjects with at least 1 hypoglycemic episode; p-values for incidences were based on a logistic regression model for Q3D vs. Q1D; due to low occurrence of severe hypoglycemia, mean ± SD and only Wilcoxon test p-values are presented Edelman et al. Diabetes Care 2014;37(8):2132-40. *p = .014 * Weight (kg) Change from Baseline (LSM SE) at 24 Weeks Weight (kg) Change from Baseline (LSM SE) at 24 Weeks AUTONOMY: Weight Change from Baseline at 24 Weeks p = .108 In both studies, subjects gained weight from baseline to endpoint (24 weeks) regardless of titration algorithm; In Study A, subjects using Q3D algorithm gained more weight from baseline than subjects using Q1D algorithm (p = .014); no significant difference in weight gain was observed between Q3D and Q1D in Study B Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Insulin Dose Study A Insulin Dose (U/d) GLA at entry, n mean (SD) Q1D (N = 267) 180 46.8 (32.4) Q3D (N = 261) 177 48.6 (27.8) Study B Q3D vs. Q1D p-value - Q1D (N = 288) 163 46.8 (29.2) Q3D (N = 290) 163 45.0 (30.0) Q3D vs. Q1D p-value - NPH at entry, n 50 48 75 78 mean (SD) 50.4 (26.7) 45.0 (26.4) 46.1 (32.7) 47.6 (23.2) Detemir at entry, n 37 35 49 47 mean (SD) 58.9 (42.6) 46.2 (30.8) 52.4 (32.0) 60.8 (44.1) ILPS at entry, n 0 0 0 1 mean (SD) NA NA NA 34.0 (NA) Basal (GLA) atrandomization, mean 62.8 (33.9) 60.3 (32.1) .335 57.3 (32.5) 60.0 (33.0) .236 (SD) Basal (GLA) at Week 24, 66.4 (35.1) 63.5 (34.6) .543 59.9 (33.4) 65.2 (42.5) .497 mean (SD) Bolus (lispro) at Week 24, 47.7 (41.1) 54.6 (46.7) .095 44.5 (36.8) 48.8 (51.0) .156 mean (SD) p-values for continuous measures were based on an analysis of variance, and categorical measures were based on Fisher’s exact test for treatment algorithm Q3D vs. Q1D Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Bolus Injections at 24-Week Endpoint Study A Study B Bolus Injections (LOCF Subjects) Q1D (N = 267) Q3D (N = 261) Q1D (N = 288) Q3D (N = 290) 1 injection, n (%) 84 (31.5) 81 (31.0) 102 (35.4) 100 (34.5) 2 injections, n (%) 69 (25.8) 66 (25.3) 85 (29.5) 89 (30.7) 3 injections, n (%) 114 (42.7) 114 (43.7) 101 (35.1) 101 (34.8) p-values were not calculated Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Discussion (Slide 1 of 2) Both algorithms (Q1D, Q3D) demonstrated statistically significant and clinically equivalent reductions in HbA1c, significant increases in 1,5-AG, and improved 7-point SMBG profiles in Studies A and B ~50% of subjects who had previously failed to reach goal HbA1c of ≤7.0% (53.0 mmol/mol) with basal insulin optimization plus OADs achieved the ADA goals for glycemic control with less glucose variability Sequential addition of prandial insulin lispro injections resulted in ~61% of subjects only requiring ≤2 doses rather than a full basalbolus regimen (ie, simplifies treatment, could enhance therapy compliance) Subjects gained 2-3 kg of weight, regardless of treatment algorithm, with the initiation of prandial insulin Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Discussion (Slide 2 of 2) Results show basal-bolus therapy can be initiated in the elderly without increased risk of hypoglycemia Regardless of titration algorithm, improved metabolic control was accomplished with low incidences and rates of nocturnal and severe hypoglycemia in both the overall study population and the elderly subgroup (≥65 years of age) with initiation and escalation of lispro Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Limitations Exclusion of subjects with BMI ≥45 kg/m2, a potentially important population with the growing healthcare burden associated with obesity Safety and efficacy need to be addressed in future research for Asian populations, as no Asian countries were included Although numerical differences were observed, which seem to benefit Q1D vs. Q3D, these were not statistically significant and should only be considered hypothesis-generating AUTONOMY algorithms were based on PK/PD modeling of GLA and lispro insulins; there may not be substantial differences with the use of other short-acting prandial insulin analogues Other combinations to control postprandial glucose excursions may be considered (such as combination of GLP-1 agonists with insulin) Edelman et al. Diabetes Care 2014;37(8):2132-40. AUTONOMY: Conclusions Trial provided novel data and basis for initiation and escalation of lispro therapy using 2 simple, self-titration regimens in patients with type 2 diabetes who failed to achieve adequate glycemic control on appropriately titrated basal insulin plus OADs Trial demonstrated a basal-bolus regimen can be initiated in an adult population, including the elderly, to lower HbA1c and limit mealtime glucose excursions safely, with either patient-driven algorithm, in endocrinology and generalist settings Utilizing Q1D and Q3D algorithms simplified insulin therapy by not requiring patient training on carbohydrate counting or insulin correction factor, and reduced the number of OADs in those treated with sulfonylurea or meglitinide Edelman et al. Diabetes Care 2014;37(8):2132-40.