* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 11, Bot 499H/505 Secondary Growth
Survey
Document related concepts
Plant physiology wikipedia , lookup
Plant stress measurement wikipedia , lookup
Gartons Agricultural Plant Breeders wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Venus flytrap wikipedia , lookup
Pollination wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Plant reproduction wikipedia , lookup
Plant morphology wikipedia , lookup
Flowering plant wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Transcript
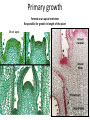
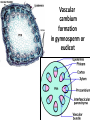
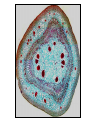
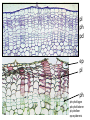
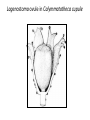
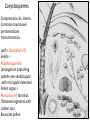
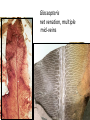
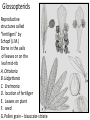
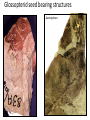
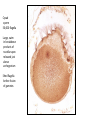
Lecture 11, Bot 499H/505 Secondary Growth Formation of the secondary xylem (=wood) and periderm (bark) systems Primary growth Formed at an apical meristem Responsible for growth in length of the plant Shoot apex Secondary growth • Cause of increased stem diameter • Arises from a lateral meristem (not at the apical meristem) • Two lateral meristems in seed plants – Vascular cambium – Phellogen = cork cambium Vascular cambium formation in gymnosperm or eudicot Vascular cambium in the stem Vascular cambium t.l.s. Pine Flowering plant Cell divisions in the vascular cambium Multiplicative = anticlinal division-adds to the width of the vascular cambium to keep up with the growth in diameter Additive = periclinal division-produces the mother cells Tilia – two year old stem Vascular rays are living parenchyma cells Salix root x.s. Primary and secondary growth Various keys and sites for wood anatomy identification • http://insidewood.lib.ncsu.edu/search • International Association of Wood Anatomists (IAWA)- List of microscopic features for hardwood identification by an IAWA Committee-Wheeler, Baas & Gasson (eds.)- 1989 • IAWA-List of microscopic features for softwood identification-Richter, Grosser, Heinz, & Gasson (eds.)- 2004 Vessel elements or no vessel elements Juniperus Ash Fraxinus Periderm Secondary tissues produced by the phellogen Protective tissue Called bark-or the bark system Consists of 3 parts Phellogen = cork cambium = lateral meristem Phellem = cork-produced to outside-cells suberized and sometimes also lignified Phelloderm = parenchyma-like-produced toward the inside-usually thin-walled cells Stages in periderm formation pl ph pd ep pl ph ph=phellogen pd=phelloderm pl=phellem ep=epidermis Fate of the epidermis and cuticle is to be sloughed off the outside of the stem as phellem produced Mesozoic Pteridosperms Subdivision: Pteridospermophytina Class: Pteridospermopsida Order: Corystospermales-Triassic-Cretaceous Order: Peltaspermales-Pennsylvanian-Triassic Order: Caytoniales-Jurassic Class: Glossopteridopsida Order: Glossopteridales- Penn.-Triassic Class: Pentoxylopsida Order: Pentoxylales-Cretaceous Lagenostoma ovule in Calymmatotheca cupule Corystosperms Compressions-So. Hemis. Common now known permineralized from Antarctica Leaf = Dicroidium (D) Seeds = Pilophorosperma (arranged on branching system-one seed/cupule with micropyle extended Pollen organ = Pteruchus (H) terminal, flattened segments with pollen sacs Bisaccate pollen Peltaspermales Pinnate leaves = Lepidopteris Seed bearing structures = Peltaspermum up to 20 seeds/ cupule on peltate head-like structures “cupulate disc” Pollen organs= on Lepidopteris foliage = Antevsia Compression/imp. So. Africa & Greenland Caytoniales Caytonia seed & cupule Caytonanthus Caytoniales Sagenopteris leaves Net-veined, compoundlike flowering plants? Gymnosperm pollinationpollen lands on micropyle of ovule-usu. carried by wind pollination droplets usually Glossopteridales • Southern Hemisphere mainly • Upper Carboniferous-Permian • The occurrence of Glossopteris leaves on all of the southern continents gave fuel to the theory of Continental Drift – Wegener 1910 • Leaves are common and in extensive matsplants that produced them were deciduous (shed their leaves) and many are found in larves (lake deposits)-so we can tell that they were shed in the fall. Glossopteris leaf mats Glossopteris Leaves have a mid-rib composed of several veins Net-venation Large leaves 10-15 cm long Spatulate Name stands for the plant and its leaf Mostly known as compression/ Impressions Permineralized material is now known from Antarctica and Australia Glossopteris net venation, multiple mid-veins Vertebraria- roots of Glossopteris Glossopterids Reproductive structures called “fertiligers” by Schopf (J.M.) Borne in the axils of leaves or on the leaf mid-rib A. Ottokaria B. Lidgettonia C. Eretmonia D. location of fertiliger E. Leaves on plant F. seed G.Pollen grain – bisaccate-striate Glossopterid seed bearing structures Austroglossa Glossopterids • Many taxa of reproductive structures (see lab manual for drawings of different types) • Pollen types include-bisaccates, monolete and trilete spores • Therefore, this is probably a large group with a lot of diversity and maybe there are several orders of plants involved here. • They all have a similar leaf and similar placement of the reproductive parts but are very different morphologically • Glossopterids also have been reported from the Jurassic of Mexico-Mexiglossa name for the leaf-little is known about this taxon. Mesozoic seed ferns • All starting to enclose their seeds in cupules of one form or another • All cupules are not equivalent, therefore the term “cupule” means different things in different groups Lyginopteridales—fused telomes-branches Caytoniales—enrolled leaf Peltaspermales—peltate head=leaf Corystospermales—enrolled leaf Mesozoic “The Age of Cycads” Subdivision: Cycadophytina (cycads) (U.Carb. ?)Permian-Recent Subdivision: Cycadeoidophytina Order: Cycadeoidales (=Bennettitales) Order: Williamsoniales (Jurassic-Cretaceous, cycadeoids) Both groups had leathery fern-like leaves Lab 15 has Mesozoic foliage types How to tell which is which? Macrozamia Australia Cycads have circinate vernation Uncoiling of leaf during development like ferns Leaves leathery= coriaceous In cycads seeds are borne on leaves=megasporophylls Cycas megasporophylls Encephalartos pollen cone Modified leaves also on the pollen cones Cycad sperm 50,000 flagella Large, swim in breakdown products of nucellar apex released just above archegonium Shed flagella before fusion of gametes Mesozoic “The Age of Cycads” Cycadeoidea Black Hills, South Dakota Collected by Cope & Marsh late 1800’s while searching for dinosaurs Trunks with mantle of protective leaf bases (sclerotic) and in the axils of leaves are the reproductive structures In Tyrell Museum of Palaeontology Monanthesia – a cone in the axil of every leaf Cycadeoidea structure studied by Wieland 1906, 1916-Yale Microsporophyll Receptacle bearing ovules and interseminal scales Burger at Field Museum Chicago Bract Structure is bisexual (contains both seeds and pollen)) Bract covered in trichomes microsporophyll Receptacle Seeds and interseminal scales Two rows of synangia on each pinna of microsporophyll Cycadeoidea reinterpreted by Delevoryas 1960’s microsporophyll bract These structures never opened like a flower How were they pollinated? Crepet 1970’s beetles seeds & interseminal scales receptacle Williamsoniella Williamsonia Cone Structure, & Receptacle Unisexual has only seeds Cycad-like foliage Ptilophyllum Pterophyllum & Ptilophyllum (small) Nilssonia Horseshoe Canyon overlook-Drumheller, AB Cretaceous Otozamites Taeniopteris Types of stomatal development in cycadophyte leaves Thomas and Bancroft 1913 Haplocheilic vs. Syndetocheilic Guard cells develop from one initial cell in leaf epidermis Subsidiary cells formed by surrounding epidermal cells Stomata in rows Epidermal cells straight-walled Cuticle thin (comparatively) Cycadales Conifers, Ephedra Ginkgo subsidiary cells develop from same initial as guard cells stomata scattered epidermal cells with wavy outlines cuticle thick Cycadeoidales Welwitschia, Gnetum Ginkgo Joffre Bridge Australia Horseshoe Canyon Fm. Drumheller Ephedra Gnetum Welwitschia