* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A Crash Course In Organic Chemistry
Survey
Document related concepts
Volatile organic compound wikipedia , lookup
George S. Hammond wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromatization wikipedia , lookup
Aromaticity wikipedia , lookup
Homoaromaticity wikipedia , lookup
Transcript
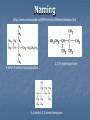
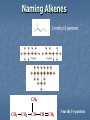
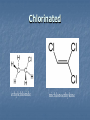
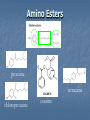
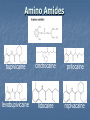
A Crash Course In Organic Chemistry Organic Chem Study of organic chemistry and life Study of organic compounds in life Study of hydrocarbon compounds in and their uses in life Alkanes (CnH2n+2) Number of Carbons Prefix Structure 1 Methane CH4 2 Ethane CH3CH3 3 Propane CH3CH2CH3 4 Butane CH3(CH2)2CH3 5 Pentane CH3(CH2)3CH3 6 Hexane CH3(CH2)4CH3 7 Heptane CH3(CH2)5CH3 8 Octane CH3(CH2)6CH3 9 Nonane CH3(CH2)7CH3 10 Decane CH3(CH2)8CH3 11 Undecane CH3(CH2)9CH3 12 Dodecane CH3(CH2)10CH3 Naming Branched Alkanes Find the longest carbon chain and name alkane Number the carbon from the end with nearest substituent (side group) Determine the name of substituent and add –yl; halogens are chloro, fluoro, iodo Put the names in alphabetical order Identify the positions of all substituents in the name by placing the carbon number where the substituent attaches to the parent chain in front of it. Arrange in alphabetical order and list each one Naming 2,2,4-trimethyl-3-propylhexane 3-ethyl-3-methyl-4,5-dipropyloctane 6-ethyl-4,5-dipropylnonane Naming http://www.sciencegeek.net/APchemistry/APtaters/alkanes.htm 4-ethyl-3-methyl-4-propyloctane 2,2,3-trimethylpentane 4,4-diethyl-2,3-dimethylheptane Reactions of Alkanes 1.Combustion: reaction with O2 in the presence of sparks Natural gas CH4(g) + 2 O2(g) disposable cigarette lighters 2 C4H10(g) + 13 O2(g) charcoal lighter fluid C5H12(g) + 8 O2(g) hydrocarbons in gasoline 2 C8H18(l) + 25 O2(g) CO2(g) + 2 H2O(g) 8 CO2(g) + 10 H2O(g) 5 CO2(g) + 6 H2O(g) 16 CO2(g) + 18 H2O(g) 2. Halogenation: reaction with halogens (I, Br, F, Cl) to form alkyl halides In the presence of light, or at high temperatures, alkanes react with halogens to form alkyl halides: light CH4(g) + Cl2(g) CH3Cl(g) + HCl(g) light CH4(g) + Br2(l) CH3Br(g) + HBr(g) Halogenation A substitution reaction where H is substituted by a halogen The reactivity of the halogens is F2>Cl2>Br2>I2 The reaction of halogen with methane or ethane will form on product since the reactivity of all of the hydrogens is exactly the same C2H6 C2H5-Cl + HCl Halogenation Halogenation of propane up to decane generate more than one alkyl halides since the Hs in the alkanes exhibit different reactivities to halogen The reactivity of the Hs: 3o >2o >1o 1o- a C bound to one C 2o- a C bound to two C H o- a C bound to three C 3 CH3-C-CH -CH3 CH3 2 Halogenation Halogenation of an alkane with more than one type of H will generate more than one alkyl halide called isomers Same chemical formula but different structures CH3-CH2-CH3 + Cl2 ——> 45% CH3-CH2-CH2Cl + 55% CH3-CHCl-CH3 CH3-CH2-CH3 + Br2 ——> 3% CH3-CH2-CH2Br + 97% CH3-CHBr-CH3 (CH3)3CH + Cl2 ——> 65% (CH3)3CCl + 35% (CH3)2CHCH2Cl Alkenes (CnH2n) Number of Carbons Prefix Structure 2 Ethene CH2=CH2 3 Propene CH2=CHCH3 4 Butene CH2=CHCH2CH3 5 Pentene CH2=CH(CH2)2CH3 6 Hexene CH2=CH(CH2)3CH3 7 Heptene CH2=CH(CH2)4CH3 8 Octene CH2=CH(CH2)5CH3 9 Nonene CH2=CH(CH2)6CH3 10 Decene CH2=CH(CH2)7CH3 11 Undecene CH2=CH(CH2)8CH3 12 Dodecene CH2=CH(CH2)9CH3 Naming Alkenes 1. Name the longest carbon chain that contains the double bond 2. The name for the alkenes ends in ene instead of –ane 3. Number the main chain from the end nearest the double bond. 4. Indicate the position of the double bond with the number of the first unsaturated carbon. 5. Place the number and names of substituents in front of the alkene name. 6. Cyclic alkenes are named as cycloalkenes. Naming Alkenes 2-methyl-2-pentene CH3 CH3 CH2 CH CH CH2 3-methyl-1-pentene Reactions of Alkenes REACTANT REACTION H2 Hydrogenation (catalyzed by metals) H2O Hydration (catalyzed by acid) X2 Halogenation HX Hydrohalogenation KmnO4, Oxidation H2O, and OH- EXAMPLE CH2=CH2 → H-CH2-CH2-H CH2=CH2 → H-CH2-CH2-OH CH2=CH2 → Cl-CH2-CH2-Cl CH2=CH2 → Cl-CH2-CH2-H CH2=CH2 → OH-CH2-CH2-OH Alkynes (CnH2n-2) Number of Carbons Prefix Structure 2 Ethyne CHΞCH 3 Propyne CHΞCCH3 4 Butyne CHΞCCH2CH3 5 Pentyne CH ΞC(CH2)2CH3 6 Hexyne CH ΞC(CH2)3CH3 7 Heptyne CH ΞC(CH2)4CH3 8 Octyne CH ΞC(CH2)5CH3 9 Nonyne CH ΞC(CH2)6CH3 10 Decyne CH ΞC(CH2)7CH3 11 Undecyne CH ΞC(CH2)8CH3 12 Dodecyne CH ΞC(CH2)9CH3 Naming Alkynes 1. Name the longest carbon chain that contains the triple bond 2. The name for the alkenes ends in yne instead of –ane 3. Number the main chain from the end nearest the triple bond. 4. Indicate the position of the triple bond with the number of the first unsaturated carbon. 5. Place the number and names of substituents in front of the alkyne name. Reactions of Alkynes REACTANT REACTION H2 Hydrogenation (catalyzed by metals) H2O Hydration (catalyzed by acid) X2 Halogenation HX Hydrohalogenation KmnO4, Oxidation H2O, and OH- EXAMPLE CHΞCH → CH2=CH2 CHΞCH → CH2=CH-OH CHΞCH → Cl-CH=CH-Cl CHΞCH → Cl-CH=CH2 CHΞCH → OH-CH=CH-OH Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered to be derivatives of hydrocarbons Organic Functional Gps Organic Compounds Containing Oxygen Many of the important functional groups in organic compounds contain oxygen Examples are alcohols ethers aldehydes ketones carboxylic acids esters Alcohols An alcohol is a compound obtained by substituting a hydroxyl group (-OH) for a –H atom on a carbon atom of a hydrocarbon group. • Some examples are CH3 OH methanol CH3 CH2 OH ethanol OH CH3 CH CH3 2-propanol Alcohols An ether is a compound with an oxygen “bridge” between two alkyl groups. • An example is CH3 CH2 O CH2 CH3 diethyl ether An aldehyde is a compound containing a carbonyl group with at least one H atom attached to it. • An example is O CH3 CH ethanal A ketone is a compound containing a carbonyl group with two hydrocarbon groups attached to it. • An example is O CH3 CH2 C CH3 2-butanone A carboxylic acid is a compound containing the carboxyl group, -COOH. • An example is O CH3 C OH ethanoic acid An ester is a compound formed from a carboxylic acid, RCOOH, and an alcohol, R’OH. • The general structure is O R C O R' Most organic bases are amines, which are compounds that are structurally derived by replacing one or more hydrogen atoms of ammonia with hydrocarbon groups. H H N R' R' R primary amine H N R secondary amine R" N R tertiary amine Amides are compounds derived from the reaction of ammonia, or of a primary or secondary amine, with a carboxylic acid. • The general formula for a common amide is R O H C N H Groupings of Organic Compounds Organic Comounds Aliphatic compounds contain straight or branched chains or rings containing single carbon bonds Aromatic compounds carbon-based rings or multi-rings with alternating single & double carbon bonds Aromatic Compounds ring compounds: bonds alternate between single & double ones (bonds actually resonate) most common is benzene when one hydrogen is replaced: name by placing the name of the substituent first, followed by -benzene when two hydrogens replaced: ortho (o-), meta (m-) or para (p-) used when more hydrogens replaced: use numbering system for positions on the Benzene benzene naphthalene Polycyclic Aromatic Hydrocarbons (PAHs) two or more benzene rings fused together, sharing pairs of carbon atoms PNAs: polynuclear aromatic compounds PCBs: Polychlorinated biphenyls PCDDs: Polychlorinated dibenzodioxins PCDFs: Polychlorinated dibenzofurans anthracene General Anesthetics Ether and Chloroform These agents are the anesthetics from hell Have negative side effects Flammable and very toxic CH3-CH2-O-CH2-CH3 Non Halogenated Hydrocarbons all of these will work, and the longer the chain, the higher the potency. However, they have a tendency to produce cardiovascular toxicity. Cyclopropane (U.S.P.) is the only one still in use, and it is explosive. Ethers Like hydrocarbons, the longer the chain, the more potent the anesthetic. However, increasing chain length also increases toxicity and reduces induction time. Ethyl ether is seldom used, and divinyl ether is explosive and produces deep anesthesia too quickly. CH3-CH2-O-CH2-CH3 Halogenated Hydrocarbons-Cl Addition of a halogen can reduce or eliminate flammability, and can also increase potency. Depending on the halogen, some of these compounds can cause arrhythmias and/or renal or hepatic toxicity. Compounds containing only bromine are generally not useful. Compounds containing only chlorine are subject to limited use, are toxic, and can cause arrhythmias. The best of the chlorinated agents are ethyl chloride and trichloroethylene Chlorinated ethylchloride trichloroethylene Halogenated-F Fluorinated hydrocarbons are the most useful of the general anesthetics Were first discovered as offshoots of the nuclear weapons program Addition of a fluorine decreases flammability, boiling point and the incidence of catecholinduced arrhythmias (these increase as the size of the halogen increases, and F is the smallest halogen). The structures of a few representative fluorinated hydrocarbon general anesthetics are shown Halogenated Chlorinated and Fluorinated (these are inhaled) halothane isoflurane sevoflurane enflurane desflurane Fluorinated Halothane, USP (Fluothane) - the first fluorinated hydrocarbon to be introduced, is a poor muscle relaxant, and has some toxicity and propensity to cause catechol-induced arrhythmias. Methoxyflurane (Penthrane) - this analog is somewhat better, but still causes some arrhythmias and other toxicity. It also causes a slow induction period. Enflurane, U.S.P. (Enthrane) - good anesthetic, but has unsatisfactory analgesia in Stage I. Isoflurane (Forane) - the best general anesthetic so far, it has no commonly observed ill effects. Nitrous Oxide This is the least toxic anesthetic It is the least potent anesthetic It causes good analgesia, but is a poor muscle relaxant. It is an NMDA receptor antagonist so prevent transmission of signals between neurons in the brain N2O Barbiturates (IV) Derivatives of barbit acid act as central nervous system depressants, produce a wide spectrum of effects, from mild Barbituric acid sedation to anesthesia Activate the GABA receptor. GABA is the M principal inhibitory neurotransmitter in the et h Na thiopental mammalian Central Nervous System (CNS).oh e Methohexital Benzodiazepines (IV) Psychoactive drugs Used before certain medical procedures such as endoscopies or dental work and prior to some unpleasant medical procedures in order to induce sedation and amnesia for the procedure Activates the GABA receptor diazepam midazolam lorazepam Propofol a short-acting intravenous anesthetic agent used for the induction of general anesthesia in adult patients and pediatric patients older than 3 years of age Activates the GABA receptor-inhibits signal transmission in the brain Etomidate short acting intravenous anesthetic agent used for the induction of general anaesthesia and for sedation for short procedures Ketamine HCl (IV) deriv of phencyclidine acts like a volatile anesthetic agent It is potent, rapid acting and has a short duration Patients older than 16 will often (27%) have wild dreams and hallucinations during emergence, and so only indicated for children less than 16 years old. NMDA receptor antagonist Local Anesthetics Local Anesthetics Local anesthetics are agents which prevent transmission of nerve impulses without causing unconsciousness. They act by binding to fast sodium channels from within (in an open state). Local anesthetics can be either ester or amide based. Local Anesthetics Have the following general structure: Aromatic-benzene ring-hydrophobic Intermediate-amide or ester portion Amino portion-hydrophyllic Amino Esters procaine tetracaine chloroprocaine cocaine Amino Amides bupivicaine cinchocaine prilocaine levobupivicaine lidocaine ropivacaine Common Local Anesthetics Duration w/o Epi, min Duration W/ Epi, min Maximum Dose w/o Epi, mg/kg Maximum Dose W/ Epi, mg/kg Cocaine 45 - 2.8 - Procaine 15-30 30-90 7.1 8.5 Chloroprocaine 30-60 - 11.4 14.2 120-240 240-480 1.4 - Lidocaine 30-120 60-400 4.5 7 Mepivacaine 30-120 30-120 4.5 7 Bupivacaine 120-240 240-480 2.5 3.2 Etidocaine 200 240-360 4.2 5.7 Prilocaine 30-120 60-400 5.7 8.5 Anesthetic Esters Tetracaine Amides