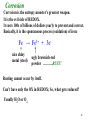* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ch19 MSJ jlm
Metallic bonding wikipedia , lookup
Marcus theory wikipedia , lookup
Transition state theory wikipedia , lookup
Water splitting wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Oxidation state wikipedia , lookup
Stoichiometry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Cathodic protection wikipedia , lookup
IsaKidd refining technology wikipedia , lookup
Chemical reaction wikipedia , lookup
Theory of solar cells wikipedia , lookup
Click chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nickel–metal hydride battery wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
History of electrochemistry wikipedia , lookup
Mercury-arc valve wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Gaseous detection device wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electrolysis of water wikipedia , lookup
Chapter 19
Electrochemistry
and Its Applications
Humphry Davy
1778-1829.
Prepared metallic K, Na, Sr,
Ca, B, Ba, Mg, Li by electrolysis.
1
Oxidation-Reduction Reactions
OXIDATION = loss of electrons
Examples:
Na Na+ + eAl Al3+ + 3eS2- S + 2e-
LEO the lion
goes GER
OXIDATION = increasing the oxidation number (more positive)
Example:
NO
NO2
Oxid. Nos: +2 -2
+4 -2
change = +2
+2 to +4 :
N is oxidized
2
Oxidation-Reduction Reactions
REDUCTION = gain of electrons
Examples:
N + 3e- N3Fe3+ +e- Fe2+
LEO the lion
goes GER
REDUCTION = decreasing the oxidation number (more negative)
Example:
MnO4- Mn2+
Oxid. Nos:
+7
+2
change = -5
+7 to +2 :
Mn is reduced
3
Review of Oxidation Numbers
O.N. = 0 for atom in element form (C, Ag, O2, H2, P4, etc.)
= charge for any monoatomic ion
(e.g., +1 for Na+, -2 for S2- , +3 for Al3+)
= -2 for oxygen in compound or ion (except peroxides
and when bonded to fluorine)
= +1 for hydrogen when bonded to non-metals (e.g.,
H2O, CH4, H3N, HCl)
= -1 for hydrogen when bonded to metals (e.g., NaH)
= -1 for oxygen in peroxides (e.g., HOOH)
Sum of O.N. = 0 for neutral compound
(e.g., for Na2CO3, Na = +1, O = -2, C = +4)
Sum of O.N. = ion charge for polyatomic ion
(e.g., for CO3-2, O = -2, C = +4)
4
Oxidation-Reduction Reactions = “REDOX”
What we have looked at are called half-reactions.
Half-reactions are fully balanced with respect to both
mass (atoms) and electrons (net charge) and are either
reduction or oxidation (but not both), e.g.,
Sn2+ + 2e- Sn
(Sn is reduced)
FeCl3 + e- FeCl2 + Cl(Fe is reduced)
Mn2+(aq) + 4 H2O (l) 8H+(aq) + MnO4-(aq) + 5e(Mn is oxidized)
But half-reactions do not occur by themselves in the
real world; reduction cannot occur without oxidation
(and vice versa).
Hence, we have REDOX occurring (both oxidation
and reduction) in a chemical reaction.
5
Oxidation-Reduction Reactions
Half reactions are combined to get REDOX reactions.
When combining,
• charges must balance (this is conservation of electrons) and
• atoms must balance (this is conservation of mass).
Half-reaction method of balancing REDOX equations
Consider the (unbalanced) reaction:
MnO4-(aq) + C2O42-(aq) Mn2+(aq) + CO2(g)
(1) Write out separate reduction and oxidation half-reactions;
(2) Add H2O and/or H+ as needed for mass balance, and add
electrons (e-) for charge balance;
(3) Multiply each half reaction by a common denominator so
that electrons (e-) will cancel;
(4) Add the half reactions and (mathematically) simplify.
6
Balancing Equations by the Method of Half-Reactions
MnO4-(aq) + C2O42-(aq) Mn2+(aq) + CO2(g)
1. The two incomplete half reactions are:
MnO4-(aq) Mn2+(aq)
C2O42-(aq) 2CO2(g)
2a. Balance 1st half-reaction:
MnO4-(aq) Mn2+(aq)
5e- + 8H+ + MnO4- Mn2+ + 4H2O
2b. Balance 2nd half-reaction:
C2O42-(aq) CO2(g)
C2O42- 2CO2 + 2e7
Balancing Equations by the Method of Half-Reactions
3a. Find common denominator so that electrons will
cancel.
5e- + 8H+ + MnO4- Mn2+ + 4H2O
C2O42- 2CO2+ 2e-
x2
x5
The common denominator is 10.
3b. Multiply equations so that electrons will cancel.
10e- +16H+ + 2MnO4- 2Mn2+ + 8H2O
5C2O42- 10CO2 + 10eThe 10 e- on each side cancel.
8
Balancing Equations by the Method of Half-Reactions
4. Add equations and simplify*:
16H+ +2MnO4- +5C2O42- 2Mn2++8H2O + 10CO2
5. Check to make sure both mass and charges balance.
Left side: 16 H, 2 Mn, 28 O, 10 C, (+16-2-10) = +4
Right side: 16 H, 2 Mn, 28 H, 10 C, +4
*In some instances H+ and/or H2O appear on both sides of the
Equation, so that simplification can be performed by
subtracting H+ and/or H2O from the equation.
9
Balancing Equations by the Method of Half-Reactions
Reactions Occurring in Basic Solution
•We use OH- and H2O rather than H+ and H2O.
•First, balance as usual, then add OH- to both sides so
that all H+ is consumed.
MnO4- (aq) + Br-(aq) MnO2 (s) + BrO3-(aq) (basic soln)
2x [ 3e- + 4H+ + MnO4- MnO2 + 2H2O]
1x [ Br- + 3H2O BrO3- + 6H+ + 6e- ]
Add half reactions:
2H+ + 2MnO4- + Br- 2MnO2 + BrO3- + H2O
Add 2OH- to both sides to remove 2H+ on left
2H+ + 2OH- + 2MnO4- + Br- 2MnO2 + BrO3- + H2O + 2OH2H2O + 2MnO4- + Br- 2MnO2 + BrO3- + H2O + 2OHH2O + 2MnO4- + Br- 2MnO2 + BrO3- + 2OH10
Oxidizing and Reducing Agents
That which is oxidized is the reducing agent;
That which is reduced is the oxidizing agent.
Consider, for example, the following REDOX reaction:
undergoing
oxidation
undergoing
reduction
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Reducing agent is oxidized.
Oxidizing agent is reduced.
Note: Reactions that are not redox include acid-base rxs. (e.g.,
HCl + NaOH H2O + NaCl); and metathesis (replacement, or
ppt.) rxs. (e.g., NaCl(aq) + AgNO3(aq) NaNO3(aq) + AgCl (s)).
11
Electrolytic Cells
There are essentially two types of cells:
(a) Voltaic (or galvanic):
spontaneous REDOX rxn → electricity
(b) Electrolytic
electricity → nonspontaneous REDOX rxn
We’ll start with spontaneous processes.
Consider copper (Cu) metal
Cu
Cu
in silver ion (Ag+) soln
What happens?
1. soln becomes blue
(production of Cu2+)
2. White whiskers grow on Cu surface
(production of metallic silver, Ag)
Ag+
12
Cu
Blue solution due to
production of Cu2+ ion
Silver whiskers (Ag)
growing on Cu metal
Ag+
What are the half reactions?
Ag+(aq) + e- Ag(s)
Cu(s) Cu+2(aq) + 2eWhat is the overall REDOX reaction?
2Ag+(aq) + Cu(s) 2Ag(s) + Cu+2(aq)
Silver ions are reduced to metal silver.
Copper metal is oxidized to cupric ions.
Obviously, the process is spontaneous
(because we observe it to happen).
13
e-
Another setup: Voltaic Cells
(external flow of electrons)
Same half reactions
Electrons flow from
Cu on left to Ag on right
Cu
Cu2+
Salt
bridge
Ag
Ag
Ag+
Cu will be oxidized to produce Cu2+ {Cu(s) Cu+2(aq) + 2e-}.
Ag+ will be reduced to produce Ag {Ag+(aq) + e- Ag(s)}.
This needs to be balanced by negative (-) charges. But how?
Add a salt bridge (inverted U tube with solution of ions, e.g.
Na+ NO3-)
Through salt bridge, anions (NO3-) can now move into left
chamber to balance Cu2+ being produced.
Similarly, Na+ will move into right compartment to replace the
Ag+ being reduced.
14
e-
(Same) Voltaic Cell
This is a complete
anode
cell (made up of
two half cells)
+
Cu
Cu2+
Anode
Cu → Cu2+(aq) + 2e(oxidation)
Salt
bridge
Ag
Ag
cathode
Ag+
Cathode
Ag+ +(aq) + e- → Ag
(reduction)
Overall redox reaction:
Cu (s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag (s)
The individual metals are the anode and cathode.
Here, Cu is the anode.
Ag is the cathode
Oxidation always takes place at the anode.
Reduction always takes place at the cathode.
Electrons always flow in the external circuit from the anode
(marked “-” to the cathode (marked “+”).
15
Voltaic Cells
(a porous barrier can be used instead of salt bridge)
16
V
Voltage of Cells
(Same Cu-Ag Cell)
anode
[Cu2+]=1.0 M
e-
+
Cu
Salt
bridge
Ag
Ag
Cu2+
Ag+
Anode
Cathode
Cu(s) → Cu2+(aq) + 2e(oxidation)
cathode
[Ag+]=1.0 M
Ag+ +(aq) + e- → Ag (s)
(reduction)
Cu (s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag (s)
If the solutions of Cu2+ and Ag+ were “standard” (i.e., 1.0 M),
and we placed a voltmeter in the electron flow line, we would
get a reading of +0.46 volt (at 25oC).
This is the standard cell potential or Eocell
(This is also called electromotive force (emf)
Why is this reaction spontaneous?
Why is the voltage 0.46 volt?
17
This electromotive series
presents data showing the
tendency of substances to
gain or lose electrons.
0.46 V
Choose any two entries;
the top one will act as the
cathode and will be
reduced; the bottom one
will act as the anode and
will be oxidized.
The standard voltage will
be the algebraic difference
between the two respective
potentials.
18
The Ag|Ag+ half-cell has a higher reduction potential than the
Cu|Cu2+ half-cell. This means that the Ag/Ag+ half-cell will more
readily undergo reduction when compared to the Cu/Cu2+ halfcell, and the Cu|Cu2+ half-cell will undergo oxidation.
The Ag|Ag+ standard reduction potential is Eored = +0.80 v.
The Cu|Cu2+ standard reduction potential is Eored =+0.34 v.
The overall cell potential is given by:
Eocell= Eored (higher) – Eored (lower)
The overall cell potential is always positive if it is spontaneous
(which it will be if the higher reaction in the table is the cathode
and the lower reaction in the table is the anode).
Standard Reduction Potentials (SRPs) are all based on and
compared to the hydrogen (H2|H+) half-cell which is assigned
Eored =0 volts by convention.
Caution: spontaneous processes have positive
voltages (E°, or V°), but negative free energies (ΔG).
19
Cell EMF – different electrodes
Standard Reduction Potentials (SRPs)
In this voltaic cell, hydrogen is the cathode and zinc is the anode.
The measured voltage is 0.76 V.
20
Hydrogen is the cathode
and the zinc is the anode.
The algebraic difference
between the standard
potentials is 0.76 V.
0.76 V
Note: EMF (electromotive
force), voltage, and cell
potential are all synonyms
21
F2 is the strongest
oxidizing agent;
F- is the weakest
reducing agent.
Li is the strongest
reducing agent;
Li+ is the weakest
oxidizing agent.
22
Using the Standard Reduction Potentials
(RSP) to Predict Spontaneity
Some metals are easily oxidized whereas others are not.
e.g., Fe is oxidized by Ni2+ but Ni is not oxidized by Fe2+.
The reaction: Fe + Ni2+
Ni + Fe2+
Fe2+ + Ni occurs, but
Ni2+ + Fe does not.
Remember, the SRP table is an Activity series (see Ch 5),
a list of metals arranged in order of ease of oxidation.*
The lower a metal is on the SRP table, the more active
that metal is, i.e., the more easily it is oxidized. Any metal
can be oxidized by the ions of elements above it.
*Caution! The Activity series of Ch 5 is in reverse order
of the SRP table of this chapter.
23
Let’s repeat the exercise using specific values of V:
The voltage of the reaction Ni + Fe2+ Ni2+ + Fe
is calculated by adding the reactions:
Ni
Ni2+ (V = +0.28)
Fe2+
Fe (V = -0.44)
The summed voltage is V = –0.16 and the reaction is
nonspontaneous.
The voltage of the reaction Fe + Ni2+
is calculated by adding the reactions:
Fe
Fe2+ (V = +0.44)
Ni2+
Fe2+ + Ni
Ni (V = -0.28)
The summed voltage is V = +0.16 and the reaction is
spontaneous.
24
Nernst Equation – for nonstandard solutions –
explains why batteries “run down”
E =
Eo
0.0592
log Q
n
As Cu2+ builds up,
the Cu(s) Cu2+(aq)
equilibrium shifts to
the left (le Châtlier’s
principle)
Also, if the Cu(s) is
completely consumed,
the reaction cannot
proceed.
As Ag+ is depleted,
the Ag+(aq) Ag(s)
equilibrium shifts to the left
(le Châtlier’s principle)
e-
+
Cu
Cu2+
Anode
Cu → Cu2+(aq) + 2e(oxidation)
Salt
bridge
Ag
Ag
Ag+
Cathode
Ag+ +(aq) + e- → Ag
(reduction)
25
Batteries
Batteries are the most practical applications of voltaic cell.
All batteries have self contained anode/cathode compartments.
All operate using the same principles already discussed.
The Classic “dry” (LeClanché) cell.
zinc
anode
Mushy paste
Of MnO2 and
NH4Cl
Overall reaction:
Zn + 2 MnO2 + 2NH4+→
Zn2++2MnO(OH)+ 2NH3
E~1.5 v.
carbon
cathode
26
Batteries
Alkaline Battery (similar to dry cell but more efficient)
Anode: (Zn cap)
Zn(s) Zn2+ (aq) + 2eCathode: MnO2, NH4Cl and C paste:
2NH4+(aq) + 2MnO2(s) + 2e- Mn2O3(s) + 2NH3(aq) + 2H2O(l)
27
Lead-Acid Battery
cathode
anode
PbO2
Sulfuric acid, H2SO4
(provides H+ ions)
Pb
Eocell = 2.04 V
At cathode:
PbO2(s) + HSO4-(aq) +3H+ +2e- → PbSO4(s) + 2H2O (l)
At anode:
Pb(s) + HSO4-(aq) → PbSO4(s) + H+(aq) + 2eOverall:
PbO2(s) + Pb(s) + 2HSO4-(aq) + 2H+(aq) → 2 PbSO4(s) + 2H2O(l)
28
Batteries
Lead-Acid Battery
Six cells in series
give a total voltage
of ~12 volts in an
automobile battery.
29
Batteries
Fuel Cells
• Direct production of electricity from fuels occurs in a
fuel cell.
• On Apollo moon flights, the H2 - O2 fuel cell was the
primary source of electricity.
• Cathode: reduction of oxygen:
2H2O(l) + O2(g) + 4e- 4OH-(aq)
• Anode:
2H2(g) + 4OH-(aq) 4H2O(l) + 4e• Total reaction:
2H2(g) + O2(g) 2H2O(l)
30
Batteries
Fuel Cells
31
Corrosion
Corrosion is the entropy monster’s greatest weapon.
It is the evil side of REDOX.
It costs 100s of billions of dollars yearly to prevent and correct.
Basically, it is the spontaneous process (oxidation) of iron:
Fe → Fe3+ + 3enice shiny
metal (steel)
ugly brownish-red
powder ……….RUST!
Rusting cannot occur by itself.
Can’t have only the OX in REDOX; So, what gets reduced?
Usually H2O or O2
32
Corrosion
Common type of “rusting” redox:
(Eo ~ 0.8 V)
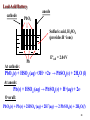
O2(g) + 4H+(aq) + 2Fe(s) → 2Fe2+(aq) + 2H2O(l)
Easy, but even more favorable in acid conditions.
There are similar equations also involving water.
Stopping Corrosion
1. Galvanize it (coat with Zn). Fe has higher SRP than
Zn. Coupled with Zn, Fe is the cathode (cathodic
protection) (look for a “matte” appearance of Zn).
2. Use “sacrificial metal” such as Mg – (this is also
cathodic protection).
3. Cover it (paint).
4. Create rust-resistant alloys, e.g., stainless steel
(Fe/Ni/Cr), or nickel steels (Fe/Ni).
33
Corrosion
Preventing the Corrosion of Iron
Also used on
ships to prevent
corrosion
34
Electrolysis
Electrolysis occurs in electrolytic cells, i.e., in cells where:
electricity → chemical reaction takes place.
This is the opposite of a voltaic cell.
external
power
In electrolysis, current is forced
into the cathode by external
power (like a battery)
cathode
anode
• Widely used for electroplating (silver, gold, copper)
• Also for production of certain metals from ores
and salts (e.g., Na, Al)
35
Electrolysis
Electrolysis of water
H2
Why is
the
volume
of H2
twice
that of
O2?
At cathode (V = 0.00):
O2
[2H+(aq) + 2e- → H2(g)] x 2
At anode (V = -1.23):
2 H2O →O2(g) + 4H+(aq) + 4eOverall:
2 H2O → 2H2(g) + O2(g)
cathode
anode
Eo = -1.23 v. --not spontaneous
external power needed 36
1.23 V
Note: EMF (electromotive
force), voltage, and cell
potential are all synonyms
37
Electrolysis – Electrolysis of Molten Salts
Decomposition of molten NaCl
Cathode: 2Na+(l) + 2e- 2Na(l)
Anode: 2Cl-(l) Cl2(g) + 2e-
Industrially, electrolysis is
used to produce metals like
aluminum (Hall-Héroult
process, where Al2O3 is
electrolyzed in molten
cryolite, Na3AlF6, with a
carbon electrode to give an
overall reaction of
2Al2O3 + 3C 4Al + 3CO2)
38
Electrolysis with Active Electrodes –
Gold plating – protects against corrosion
external power source
external power source
Au
Au
Au+(aq)
cathode:
Au+(aq) +e-→Au
Au+(aq)
anode:
Au→Au+(aq) +e-
39
40