* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Galvanic cell:
Survey
Document related concepts
Signal transduction wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cell encapsulation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell membrane wikipedia , lookup
Endomembrane system wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Transcript
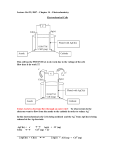
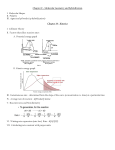
Galvanic cell: • Galvanic cell is an electrochemical device that derives electrical energy from RedOx reactions taking place within the cell. It consists of two half‐cells (metals immersed in solutions of their salts). Solutions of half‐cells are connected by a salt bridge or separated by a porous membrane and the electrodes themselves are connected by wire. The voltage is measured by voltmeter attached to the wire. • Oxidation reaction takes place on anode, reduction on cathode. • The standard electrical potential of the cell is calculated by summing the standard oxidation and reduction reactions’ potentials. Standard reduction potential of a specie depends on sublimation, ionization and hydration enthalpies, it is measured at standard conditions against SHE (E°SHE = 0,0 V). Higher is the standard potential of specie; higher is its tendency to be reduced. Daniell element: In the Daniell element, copper and zinc electrodes are immersed in a solution of copper (II) sulphate and zinc sulphate respectively (Zn‐half cell and Cu‐half cell) Oxidation: the anode half‐cell reaction: Zn(s) → Zn2+(aq) + 2e‐ Eox0 = ‐0.762 V Reduction: the cathode half‐cell reaction: Cu2+(aq) + 2e‐ → Cu(s) Ered0 = +0.339 V Overall cell reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s). E0cell = Ered0 + Eox0 = 0.339V ‐ (‐0.762V) = 1.101 V Relation between E°cell, ΔrG° and K: ΔrG° = –RT ln(K) = –nFE°cell ● For spontaneous reaction: ΔrG° ‹ 0; E°cell › 0 ● For nonspontaneous reaction: ΔrG° › 0; E°cell ‹ 0 Fuel cell: A fuel cell is an electrochemical device that converts a fuel (H2, hydrocarbons or alcohols) into an electric power by reacting it with oxidant (usually O2). The basic physical structure consists of electrolyte sandwiched between anode and cathode. At the anode, a continuous supply of fuel is being oxidized; resulting in formation of positively charged ions and negatively charged electrons. The electrolyte is designed so that ions can pass through it, but electrons cannot. Consequently, while ions travel through electrolyte directly to cathode, electrons are forced to travel through a wire connected to the anode and form electric current. After travelling the external circuit electrons reach the cathode and reunite with ions, then two react with an oxidant to create water or CO2. Tasks: 1. What is standard reduction potential? Why lithium has so low reduction potential? 2. Calculate the equilibrium constant for the Daniell cell given above. (Assume standard cond.) Reference: 1. P. Atkins, T. Overton, J. Rouke, M. Weller, F. Armstrong, (2010). Shriver&Atkins’ Inorganic Chemistry. New York, NY: Oxford University Press.