* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download handout
Survey
Document related concepts
Phosphorylation wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Cell nucleus wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell culture wikipedia , lookup
Programmed cell death wikipedia , lookup
Cellular differentiation wikipedia , lookup
Signal transduction wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Transcript
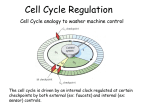
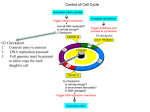
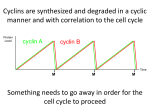
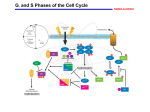
0023/0110: DNA & disease 2004-05 Cell cycle and cancer Objectives: The material covered in this lecture should enable you to Understand the general mechanisms involved in cell cycle control and its deregulation in cancer Understand how cell cycle is regulated in yeast and multicellular organisms Cancer as a result of deregulated cell proliferation: Beneath the complexity of every cancer lies a limited number of 'critical' events that have propelled the tumour cell and its progeny into uncontrolled expansion and invasion. One of these is deregulated cell proliferation, which, together with the suppression of apoptosis needed to support it, provides a minimal 'platform' necessary to support further neoplastic progression. Pathways that restrict this proliferative response in normal cells is perturbed in most cancers. One such pathway targets the principal late-G1 cell-cycle checkpoint regulated by cyclin / cdk (cyclin dependent kinases) systems. This checkpoint signalling pathway arrests the cell cycle when genomic integrity is threatened, preventing the transmission of genetic mutations into subsequent cell generations. The restriction point also regulates cell cycle progression based on environmental signals (growth factors, extracellular matrix attachment, cell–cell contacts etc). Most malignant cancers possess mutations in one or more checkpoint genes and are genetically unstable Cell cycle control The core components of the eukaryotic cell cycle engine are cyclin-dependent protein kinases (Cdks) and their regulatory subunits (cyclin). Higher eukaryotes have more forms of both cyclins and Cdks compared to lower eukaryotes. Cyclin–Cdk complexes fall into three general classes that have different substrate specificities and therefore initiate different cell cycle events 3 main classes of cyclins 1) G1 cyclins: G1 cyclin–Cdk complexes are important for progression through G1 phase and commitment to S phase; 2) S cyclin: S cyclin–Cdk complexes are responsible for initiating and completing DNA replication; 3) mitotic cyclins: M cyclin–Cdk complexes drive eukaryotes into mitosis and restrain reentry into G1 phase. Cyclin–Cdk activity is modulated by the following mechanisms 1. Cyclin availability. Association with a cyclin is absolutely required for Cdk activity. Cyclin levels can be changed by transcriptional regulation and/or by ubiquitin-dependent proteolysis. 2. Stoichiometric inhibition. Assembled cyclin–Cdk complexes can be kept inactive by stoichiometric inhibitors (cyclin kinase inhibitors; CKIs), which form inactive trimers (CKI–cyclin– Cdk). Lower eukaryotes possess a single CycB–Cdk1 specific inhibitor, whereas higher eukaryotes utilize many inhibitors in the INK4a(p15/16/17), Cip1(p21) and Kip1(p27) families. 3. Inhibitory phosphorylation. Cyclin–Cdk complexes can also be inactivated by phosphorylation of tyrosine and threonine residues close to the active site of the Cdk subunit. This phosphorylation is mediated by Wee1-type protein kinases, and the inhibitory phosphate groups are removed by Cdc25-type phosphatases. Dr. MV Hejmadi 0023/0110: DNA & disease 2004-05 Cell cycle control in yeast Regulated by size : maintained by length of cell cycle at G1 &G2 checkpoints shape: differences in phases of 2 types of yeast correlated with spindle structure genes: 1) cdc gene family (~70 known) : allows passage through checkpoint 2) wee gene family: inhibits passage thru checkpoint, encodes regulatory proteins Fission yeast (Schizosaccharomyces pombe) Size checkpoint located at G2. M-phase promoting factor (MPF) formed by cyclin B and cdc2 Cdc2 (protein kinase) drives cell into M Wee1, cdc25 phosphatase & cdc13 (cyclins) regulate function of cdc2 MPF activity regulated by inhibitory phosphorylation Cdc2 + cyclin B = inactive MPF MPF kept inactive by wee1kinase, which phosphorylates cdc2 on Y15, and MO15 kinase which phosphorylates T160. Mitosis is initiated when phosphate on Y15 is removed by cdc25 phosphatase and the balance of nuclear cdc25 exceeds that of wee1. In response to DNA damage, cells are prevented from entering mitosis by increasing Y15 phosphorylation, keeping MPF switched off leading to cell cycle arrest. cdc25 phosphatase is removed from its substrate cdc2 and exported to the cytoplasm, thereby stopping cells from entering mitosis. This export is mediated by Rad24 (attachable nuclear export sequence) Reference: Nature (1999) 397: 104-105 Cell cycle control in multicellular organisms Growth & division regulated independently unlike yeast. At least 9 cdk’s identified so far of which 5 are active during the cell cycle viz: G1(DK2,4,6), S(CDK2) & M(CDK1) & multiple cyclins A,B,C, D, E, F Uncontrolled proliferation regulated by CDK1 1) External factors: These include growth factors like PDGF & EGF, which are involved in activation of either early response genes (myc, fos & jun) or late response genes (cdks & most cyclins) or anchorage dependent factors like integrins. 2) Tumour suppressor genes that encode proteins that normally inhibit cell division. e.g retinoblastoma (Rb) gene, P53 gene,BRCA1 etc 3) Cdk inhibitors 2 families identified 1) INK4 family (inhibitor kinase4) e.g. p15, p16, p18, p19 bind specifically to CDK4 & CDK6, inhibiting cyclin D activity 2) KIP/CIP family (kinase inhibitor protein/Cdk interacting protein) e.g. p27, p57 and p21; bind & inhibit most CDK-cyclin complexes (esp G1 cyclins) 4) Oncogenes: Genes known as proto-oncogenes code for proteins that stimulate cell division; mutated forms, called oncogenes, cause stimulatory proteins to be overactive, with the result that cells proliferate excessively.e.g. genes for transcription factors like c-myc General reading: MBoC by Alberts et al (4th ed): pgs 863-906 OR Cancer Biology by RJB King Optional reading:1) Cell Proliferation (June 2003) 36(3)pp131 - The cell cycle: a review ……. targets in cancer 2) Cell cycle by Gary S Stein et al www.els.net Dr. MV Hejmadi