* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Assignment 5 Hydrogen – The Unique Element
Coordination complex wikipedia , lookup
History of molecular theory wikipedia , lookup
Computational chemistry wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Organic chemistry wikipedia , lookup
Flux (metallurgy) wikipedia , lookup
Halogen bond wikipedia , lookup
Properties of water wikipedia , lookup
Electrochemistry wikipedia , lookup
Green chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydrogenation wikipedia , lookup
Metal–organic framework wikipedia , lookup
Hydrogen isotope biogeochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Catalytic reforming wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Hydrogen peroxide wikipedia , lookup
Hydrogen sulfide wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Atomic theory wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Biohydrogen wikipedia , lookup
Hydrogen bond wikipedia , lookup
Electrolysis of water wikipedia , lookup
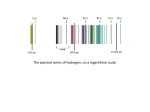
Chem 2210 Introductory Inorganic A. N. Other MUN# 200XXXXXX Assignment 5 Hydrogen – The Unique Element Elemental Hydrogen and its reactivity Although hydrogen is the simplest of all the elements, it possesses a very diverse chemistry.1 It forms compounds with nearly every other element in the periodic table! As it does not belong to a particular group, it is difficult to draw comparisons between it and other elements. It exists in its elemental form as a diatomic gas, which is Hydrogen was discovered by the British scientist Henry Cavendish in 1766. The name comes from the two Greek words "hydro" and "genes" meaning "water" and "generator".9 highly explosive and flammable in air, Figure 1. Interestingly, the cores of the largest planets in our solar system – Jupiter and Saturn – are made up of hydrogen under extreme (very high pressure) conditions.2 Scientists think that under these conditions hydrogen is metallic in nature and therefore, these planets have magnetic fields similar to earth’s although earth’s magnetic field is due to its iron core. Of course on earth, hydrogen is a non-metal. Figure 1: Colourised photograph of the Hindenburg (hydrogen-filled zeppelin) burning in 1937.3 Equation for burning of hydrogen added by author. [Note: Image reproduced without copyright permission] H2 + 1/2 O2 'spark' H2 O Hydrogen does not exist stably in its atomic form but can exist as both H+ (known as a proton) or H- (the hydride ion). There are two less common isotopes of hydrogen (1H) that have a higher mass – they are heavier isotopes – and are called deuterium (2H or D) and tritium (3H or T).4 Deuterium is of interest to chemists as it can be used to make ‘labelled’ compounds, which are used in NMR spectroscopy.* Tritium is formed through nuclear reactions in the upper atmosphere. It is the most rare isotope of hydrogen and is radioactive. * NMR spectroscopy is used widely in synthetic chemistry to obtain structural information on new compounds e.g. new medicines and plastics. In most cases, the new compound is dissolved in a deuterated solvent because this stops the 1H NMR spectrum becoming ‘swamped’ by signals from 1H present in the solvent. [Box 2.4, Housecroft and Sharpe, 2nd edition, pages 66-67] In short, in NMR experiments, we want to see just the 1H signals from the new compound and not from the solvent dissolving it. 1 Chem 2210 Introductory Inorganic A. N. Other MUN# 200XXXXXX Hydrogen can be made in a number of ways, equations 1-4.5 On a small scale, it can be formed through the reaction of an active metal with an acid, equation 1. That is, reaction of hydrochloric, sulfuric or nitric acid with a metal that have a negative standard potential e.g. Zn2+/Zn Eo –0.76 V, Cr3+/Cr Eo –0.74 V, Fe3+/Fe Eo –0.44 V.6 On a larger scale and in industry, hydrogen is produced via a process known as steam reforming, equation 2. Industry is also interested in using coal to produce H2 industrially, as coal is cheaper than methane and easier to transport, equation 3. Both equations/processes 2 and 3 require high temperatures and catalysts to generate H2. This is probably a reflection of the immense thermodynamic stability of water (the source of hydrogen in these reactions). Zn(s) + H2SO4(aq) → Zn2+(aq) + SO42-(aq) + H2(g) (equation 1) CH4(g) + H2O(g) → CO(g) + 3 H2(g) (equation 2) C(s) + 2 H2O(g) → CO2(g) + 2 H2(g) (equation 3) H2O(l) → H2(g) + ½ O2 (g) Ecello –1.23 V (equation 4) Many researchers are interested in forming hydrogen through electrolysis, equation 4, as renewable energy e.g. solar-power could be used and lead to a clean fuel for transport as water is reformed as the only by-product, Figure 2. Figure 2: Simple schematic representations of the hydrogen cycle and a hydrogen fuel cell.7,8 [Note: Image reproduced without copyright permission] Hydrogen can be used in many other areas, in addition to its use as a clean fuel, as detailed in the bullet list on the next page:9,10 2 Chem 2210 Introductory Inorganic A. N. Other MUN# 200XXXXXX • Fixation of nitrogen in the Haber process to produce ammonia.† • Production of hydrochloric acid. • Manufacture of margarine via hydrogenation of oils and fats. Note: this is a reduction process. Double C=C bonds are converted to single C-C bonds. • Production of metals through reduction of metallic ores. o • As can be seen from the above two points (and in methanol synthesis below) – Hydrogen is a very important reducing agent. It is an important feedstock in methanol production. CO(g) + 2 H2(g) → CH3OH(g), which requires a catalyst and is performed at high temperature.10 o Methanol in turn is a feedstock for other processes. • As a rocket fuel! • In welding - ‘atomic hydrogen welding’ can be used to melt and weld tungsten metal.11 Tungsten is the metal with the highest melting point known and cannot be melted using conventional means. • In cryogenics, as liquid hydrogen, because it has a melting point only just above absolute zero. Note: Helium is used more commonly because of the flammability of hydrogen. H In coordination compounds, hydrogen is known as dihydrogen and can act as a Lewis base by donating some of the electron density from its H-H σ bond to a M Lewis acidic metal centre, Figure 3.12 H Figure 3: Schematic diagram of a metal bonding with dihydrogen Compounds of hydrogen and their reactivity For this part of the essay, I will focus on binary compounds of hydrogen i.e. they will only contain hydrogen and one other element. Compounds of hydrogen fit into three categories:13 1. Molecular hydrides e.g. methane CH4, ammonia NH3 and water H2O 2. Saline hydrides e.g. LiH and CaH2 3. Metallic hydrides e.g. TiHn and NiHn Molecular hydrides normally contain a p-block element with similar or higher electronegativity compared with hydrogen. Binary molecular hydrides are often gases. Water is an exception because of † Ammonia is of crucial importance to world food production and this process uses the most hydrogen on a yearly basis! 3 Chem 2210 Introductory Inorganic A. N. Other MUN# 200XXXXXX hydrogen-bonding. The number of possible binary hydrides is expanded significantly if we consider negatively and positively charged species, Table 1. Saline hydrides can be regarded as salts containing H- ions and are crystalline in nature. They are formed by electropositive metals i.e. group 1 and 2 metals. Table 1:An array of some isoelectronic hydrides of the 2s22pn elements14 Number of Hs 4 3 2 1 Group 13 BH4BH32- Group 14 CH4 CH3- Group 15 NH4+ NH3 NH2- Group 16 H3O+ H2O OH- Group 17 H2F+ HF Both molecular and saline hydrides are quite reactive. Group 1 and 2 hydrides react vigorously with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have different reactivities depending on their group and also the first row 2p compounds are significantly different to those formed with elements further down the periodic table. For example, the halogens form acidic hydrides e.g. HCl, but it should be noted that HF is anomalous in that it is a weak acid. Hydrides of the pnictogens are all Lewis bases and useful ligands in coordination chemistry. However, in contrast to NH3, PH3 and AsH3 are very toxic and rarely used. Also, PH3 and AsH3 are readily oxidized to give pentavalent central atoms e.g. O=PH3, whereas NH3 is stable in air. Ammonia dissolves in water to give ammonium hydroxide solutions, whereas PH3 is not very soluble in water. This is likely a result of the hydrogen-bonding that is possible for 2p molecular hydrides of electronegative elements i.e. NH3, H2O and HF. Hydrides of group 13 – B, Al, Ga – are electron deficient and therefore, exist as dimers e.g. B2H6. They are also Lewis acids and form adducts with Lewis bases. As mentioned above hydrogen-bonding plays an important role in the physical properties of many molecular hydrides e.g. boiling points, pKa. It is also important in many biological settings e.g. base-pairing in DNA. Typically, hydrogen bonds have enthalpies of between 20 and 60 kJ mol-1.15 The presence of hydrogen bonds within certain compounds can be detected using molecular structure determination (X-ray crystallography and neutron diffraction studies), infrared spectroscopy (where bands become broadened if hydrogen-bonding is present) and 1H-NMR (where unusual proton chemical shifts will be seen).15 An important manifestation of hydrogen-bonding, with particular relevance to Newfoundland, is the structure of ice. The hydrogen-bonding leads to ice having a lower 4 Chem 2210 Introductory Inorganic A. N. Other MUN# 200XXXXXX density than liquid water and therefore, ice can float on water. Without hydrogen-bonding, we would not see icebergs off the coast of this province in May and June. Figure 4: Icebergs trapped in Quidi Vidi harbour, image taken without permission from http://www.canadian-travel.ca/ Current research surrounding the chemistry of hydrogen As mentioned earlier in this essay, there is immense interest in hydrogen fuel cells as a source of clean energy. In November 2010, Mitch Jacoby wrote a short report in a chemical newspaper on a nickel complex that could boost hydrogen oxidation.16 A team of US researchers at the Pacific Northwest National Laboratory and Villanova University made a Ni2+ coordination compound where the metal centre was bonded to two bidentate chelating phosphine ligands. They found that it was an excellent electrocatalyst for oxidizing hydrogen.17 This is important because expensive platinum metal is currently used as the electrocatalyst in hydrogen fuel cells, and nickel is much cheaper. Electrocatalytic hydrogen oxidation is when hydrogen is separated into protons and electrons and forms water upon reaction with oxygen. The experiments that the researchers performed included X-ray crystallography, cyclic voltammetry and computational studies. They concluded their paper by saying that their electrocatalyst “is 5 times faster than the best molecular H2 oxidation catalyst previously reported.” We can only hope that advances will continue to be made in this field and soon we will have cheaper hydrogen fuel cells and clean energy for all. References 1) Shriver & Atkins’ Inorganic Chemistry, P. Atkins, T. Overton, J. Rourke, M. Weller, F. Armstrong and M. Hagerman, W. H. Freeman and Company, New York, 5th Edition, 2010, Chapter 10, Hydrogen, pages 274-279. 2) Inorganic Chemistry, C. E. Housecroft and A. G. Sharpe, Pearson Education Ltd., Harlow, 2nd Edition, 2005, Chapter 9, Hydrogen, Box 9.1, page 239. 3) Picture taken from http://depletedcranium.com/what-killed-the-hindenburg-hydrogen-or-fabric/ accessed January 11th 2011. 5 Chem 2210 Introductory Inorganic A. N. Other MUN# 200XXXXXX 4) Basic Inorganic Chemistry, F. A. Cotton, G. Wilkinson and P. L. Gaus, John Wiley & Sons Inc., New York, 3rd Edition, 1995, Chapter 9, Hydrogen, page 273. 5) Shriver & Atkins’ Inorganic Chemistry, P. Atkins, T. Overton, J. Rourke, M. Weller, F. Armstrong and M. Hagerman, W. H. Freeman and Company, New York, 5th Edition, 2010, Chapter 10, Hydrogen, Section 10.4, Production of dihydrogen, pages 280-281. 6) Shriver & Atkins’ Inorganic Chemistry, P. Atkins, T. Overton, J. Rourke, M. Weller, F. Armstrong and M. Hagerman, W. H. Freeman and Company, New York, 5th Edition, 2010, Standard potentials taken from Resource Section 4, pages 787-799. 7) Picture taken from http://kuzwe.com/hydrogen_clean.asp accessed January 11th 2011. 8) Picture taken from http://images.yourdictionary.com/images/computer/_FUELCEL.GIF accessed January 11th 2011. Note: Image prepared by Ballard power systems, a leading company in fuel cell technologies based in Vancouver, BC. 9) http://www.webelements.com/hydrogen/history.html and http://www.webelements.com/hydrogen/uses.html accessed January 10th 2011. 10) Inorganic Chemistry, C. E. Housecroft and A. G. Sharpe, Pearson Education Ltd., Harlow, 2nd Edition, 2005, Chapter 9, Hydrogen, pages 238-239. 11) http://en.wikipedia.org/wiki/Atomic_hydrogen_welding accessed January 10th 2011. 12) Basic Inorganic Chemistry, F. A. Cotton, G. Wilkinson and P. L. Gaus, John Wiley & Sons Inc., New York, 3rd Edition, 1995, Chapter 9, Hydrogen, page 282. 13) Shriver & Atkins’ Inorganic Chemistry, P. Atkins, T. Overton, J. Rourke, M. Weller, F. Armstrong and M. Hagerman, W. H. Freeman and Company, New York, 5th Edition, 2010, pages 276-277. 14) Personal Communication, Geoff Rayner-Canham, Memorial University of Newfoundland, December 2009. 15) Shriver & Atkins’ Inorganic Chemistry, P. Atkins, T. Overton, J. Rourke, M. Weller, F. Armstrong and M. Hagerman, W. H. Freeman and Company, New York, 5th Edition, 2010, pages 284-286. 16) M. Jacoby, Chemical & Engineering News, 2010, 88, Iss. 47 (November 22), p. 28. 17) Jenny Y. Yang, S. Chen, W. G. Dougherty, W. S. Kassel, R. M. Bullock, D. L. DuBois, S. Raugei, R. Rousseau, M. Dupuis and M. Rakowski DuBois, Chemical Communications, 2010, 46, 8618-8620. 6