* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download OPTIMISING GENE TRANSFER INTO EMBRYONIC KIDNEYS AS A
Genetic engineering wikipedia , lookup
Point mutation wikipedia , lookup
Genomic imprinting wikipedia , lookup
Gene expression programming wikipedia , lookup
Gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Oncogenomics wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
History of genetic engineering wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genome (book) wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Designer baby wikipedia , lookup
Gene expression profiling wikipedia , lookup
Microevolution wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
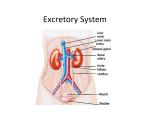
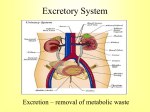
OPTIMISING GENE TRANSFER INTO EMBRYONIC KIDNEYS AS A PRELUDE TO TREATING RENAL TRACT MALFORMATIONS. INTRODUCTION. Renal tract malformations can be devastating, accounting for about half of children, and a large subset of young adults, with end stage renal disease. Most are identified at fetal ultrasound screening and definition of their genetic pathogenesis is making it possible to conceive of novel biological therapies. We hypothesised that viruses can be used to transfer genes into embryonic kidneys. Accordingly, our aim was optimise efficient gene transfer without compromising normal nephron differentiation. METHODS. We isolated wild type embryonic day 13 mouse kidneys, anatomically equivalent to mid first trimester human kidneys. Each organ was comprised of a branching ureteric tree and nephron precursors. Kidneys were exposed to replication defective vesicular stomatitis virus glycoprotein (VSVg) pseudotyped lentiviruses expressing green fluorescent protein (GFP) from either a cytomegalovirus (CMV) or a 3-phosphoglycerate kinase (PGK) promoter. Kidneys were grown in organ culture for up to three days and transgene expression visualised as green fluorescent cells. RESULTS. Intact kidneys, unexposed to virus, differentiated in culture to form Ecadherin+ collecting ducts and Wilms tumour 1+ glomeruli. When intact rudiments were exposed to lentivirus, differentiation was also good but few if any cells expressed GFP. Reasoning that the virus could not penetrate into the organ, we dissociated embryonic kidneys into single cells, exposed them to virus, and then placed the cell pellet into organ culture. Remarkably, even after being dissociated for one hour, the pellet rapidly moulded itself into a kidney shape and then differentiated nearly as well as intact controls. After dissociated cells were exposed to virus, efficient gene transfer and strong GFP expression from each promoter occurred in the reconstituted organ. A ratio of adding one to five viral particles per kidney cell provided the best balance between strong transgene expression without compromising nephron differentiation. CONCLUSION. Lentiviruses target embryonic kidney cells and the transduced reporter gene is robustly expressed. Further studies are now needed to optimise ex vivo and in vivo transfer of reporter, and then therapeutic, genes into embryonic kidneys from mice with mutations of human renal tract malformation genes.