* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PREVALENCE OF RELATIVE BRADYCARDIA IN ORIENTIA
Survey
Document related concepts
Leptospirosis wikipedia , lookup
Neonatal infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Hepatitis B wikipedia , lookup
Typhoid fever wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
Rocky Mountain spotted fever wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
1793 Philadelphia yellow fever epidemic wikipedia , lookup
Transcript
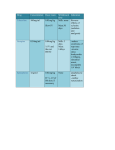
Am. J. Trop. Med. Hyg., 68(4), 2003, pp. 477–479 Copyright © 2003 by The American Society of Tropical Medicine and Hygiene PREVALENCE OF RELATIVE BRADYCARDIA IN ORIENTIA TSUTSUGAMUSHI INFECTION DAVID M. ARONOFF AND GEORGE WATT Divisions of Infectious Diseases and Pulmonary and Critical Care Medicine, Department of Medicine, University of Michigan Health System, Ann Arbor, Michigan; Retrovirology Department, United States Army Military Component, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand Abstract. We investigated 100 febrile patients infected with Orientia tsutsugamushi (the etiologic agent of scrub typhus) for the presence of relative bradycardia, defined as in increase in heart rate of < 10 beats/minutes/1°C increase in temperature. The median heart rate response for the entire febrile scrub typhus population was 9.3 beats/minute/°C and the prevalence of relative bradycardia was 53%. The occurrence of relative bradycardia was independent of patient age or gender. There were no differences in median basal temperature or febrile temperature between those patients exhibiting relative bradycardia and those with a normal febrile pulse increase. However, febrile patients with relative bradycardia had a significantly higher resting pulse rate following recovery from infection than did patients who had a normal pulse increase during their illness. These data demonstrate that relative bradycardia frequently accompanies mild infection with O. tsutsugamushi and that baseline cardiovascular parameters may affect the febrile heart rate response to scrub typhus. ferral center. Patient selection was performed as previously described.16 Eligible patients were ambulatory adults without hypotension, shock, impaired consciousness, or pulmonary dysfunction. Additional exclusion criteria were vomiting, presence of a febrile illness other than infection with O. tsutsugamushi, a serum bilirubin concentration >25.7 mol/L, an alanine aminotransferase concentration >100 units/L, and ingestion of acetaminophen, nonsteroidal anti-inflammatory drugs, chloramphenicol, tetracycline, or ciprofloxacin during the 48-hour period before evaluation. No patients were receiving treatment with beta-blockers. Study participants gave written informed consent according to a protocol approved by the U.S. Army Scientific and Human Use Review and Regulatory Affairs Division and by the Thai Ministry of Public Health Human Use Committee. Data acquisition. Oral temperature and heart rate data were recorded upon presentation, during antibiotic treatment, and following symptom resolution. Fever was defined as an oral temperature ⱖ37.8°C. Febrile heart rate and temperature data represent the vital signs documented on initial patient evaluation, before the application of antibiotic therapy. Baseline temperature and heart rate were assessed when patients first became and then remained afebrile following treatment. Although no uniform definition of relative bradycardia exists, we defined it a priori as an increase in the heart rate from a baseline of <10 beats/minute/1°C increase in temperature.7 Patients exhibiting a pulse increase ⱖ10 beats/minute/°C were classified as having a normal pulse increase (NPI). Infection with O. tsutsugamushi was diagnosed serologically by a dot-blot rapid enzyme-linked immunosorbent assay (DipSticks; Integrated Diagnostics, Baltimore, MD) and confirmed by an indirect immunoperoxidase test if IgM antibody titers were >1:400 or IgG titers were >1:1,600. Statistical analysis. Data are expressed as the median (interquartile range [IQR]). Comparison of continuous variables between the NPI and relative bradycardia groups was performed using a Mann-Whitney rank sum test. A chi square analysis was used to compare independent proportions between groups. Statistical analyses were performed with Prism 3.0 software (GraphPad Software, San Diego, CA). A P value of 0.05 was considered statistically significant. INTRODUCTION During infection, there tends to be a close degree of relationship between pulse rate and temperature.1,2 The usual heart rate increase in response to microbial invasion or exogenously administered, noninfectious pyrogens ranges from 10 to 18 beats/minute/1°C increase in temperature, although there is considerable individual variation.1,3−6 Relative bradycardia, an unexpectedly low heart rate response for a given increase in temperature, has been reported as a feature of a number of infectious diseases, particularly those caused by intracellular gram-negative organisms. This pulse-temperature dissociation has been associated with typhoid fever, Legionnaire’s disease, babesiosis, Q fever, infection with Rickettsia sp., malaria, leptospirosis, pneumonia caused by Chlamydia sp., and viral infections such as dengue fever, yellow fever, and viral hemorrhagic fevers.7−10 Neither the microbial and/or host factors responsible for a relatively slow pulse response to infection nor the clinical significance of relative bradycardia are known. Orientia tsutsugamushi, the causative agent of scrub typhus, is a gram-negative intracellular pathogen.11 Scrub typhus is a common zoonotic disease of rural Asia and the Western Pacific islands, transmitted by infected larval trombiculid mites, and characterized by general malaise, fever, headache, myalgias, conjunctival suffusion, cough, rash, regional lymphadenopathy, and eschar formation. Relative bradycardia is mentioned in reports of scrub typhus case series from the preantibiotic era,12,13 but it was not the primary focus of these reports and was not quantified. A description of scrub typhus in 32 cases contracted during the Vietnam War noted a prevalence of 97%, but a definition of relative bradycardia was not provided.14 In a somewhat more recent report, relative bradycardia was said to occur in 40% of the cases, again without a specific definition, and no details were given.15 We therefore prospectively examined 100 febrile patients with documented scrub typhus to further define the relationship between heart rate and temperature in this condition. MATERIALS AND METHODS Study population. The study was conducted in northern Thailand at Chiangrai Regional Hospital, a tertiary-care re- 477 478 ARONOFF AND WATT TABLE 1 Characteristics of 100 patients with scrub typhus* Patient characteristic Normal pulse increase (n ⳱ 47) Relative bradycardia (n ⳱ 53) P Age (years) Males (%) Basal temperature (°C)† Febrile temperature (°C) Basal heart rate (beats/minute)† Febrile heart rate (beats/minute) 32 (25–39) 53 36.9 (36.7–37.1) 38.9 (38.7–39.6) 66 (61–76) 100 (89–114) 33 (27–50) 55 36.8 (36.6–37.1) 39.0 (38.4–39.7) 75 (65–84) 88 (80–96) 0.21 0.88 0.30 0.65 0.04 <0.0001 * Values are the median (interquartile range) unless otherwise indicated. † Baseline temperature and heart rate were assessed when patients first became and then remained afebrile following treatment. RESULTS Pulse and temperature changes provoked by infection with O. tsutsugamushi. The dynamic relationship between heart rate and an increase in temperature was assessed in 100 febrile patients infected with O. tsutsugamushi. The characteristics of these patients are summarized in Table 1. The age range was 18−68 years and 54% were men. Fifty-three patients (53%) responded to fever with a heart rate increase ⱕ10 beats/minute/°C (relative bradycardia group), the majority of whom (62%) did not mount a response greater than 7.5 beats/minute/°C. The remaining 47 patients had a heart rate response ⱖ10 beats/minute/°C (NPI group). The median (IQR) heart rate response for the entire febrile scrub typhus population was 9.3 (6.3−12.5) beats/minute/°C. Interestingly, 65% of the entire O. tsutsugamushi-infected population had a febrile heart rate <100 beats/minute on presentation. Basal (resting) and febrile temperatures were not significantly different between patients exhibiting relative bradycardia or NPI. Although patients exhibiting relative bradycardia had a higher median resting heart rate than did patients who responded to scrub typhus with a normal heart rate response (75 versus 66 beats/minute; P ⳱ 0.04), the opposite effect was seen during fever, with patients with relative bradycardia achieving significantly lower heart rates than the NPI cohort (88 versus 100 beats/minute; P < 0.0001) (Figure 1A). Despite differences in the resting heart rate between the relative bra- dycardia and NPI patients, there was no significant linear relationship between resting heart rate and the cardiac response to infection (R2 ⳱ 0.10; Figure 1B). An analysis of patients with higher degrees of fever (temperature ⱖ38.9°C) showed that the median peak temperatures in the NPI (n ⳱ 30) and relative bradycardia (n ⳱ 27) groups were 39.3 (38.95−39.85)°C and 39.6 (39.20−40.00)°C, respectively (P ⳱ 0.11). The median heart rate response for these 57 patients was 10.0 (6.9−13.0) and the prevalence of relative bradycardia in this subgroup remained high (47%). DISCUSSION Our findings support previous reports that relative bradycardia occurs in scrub typhus12−15 and quantify its frequency and magnitude. In this study of 100 serologically documented patients with O. tsutsugamushi infections, we found that the prevalence of relative bradycardia was 53%, a feature independent of patient age or gender. In addition, the majority of the patients with scrub typhus had a heart rate <100 beats/ minutes on presentation with pyrexia. It has been suggested that the term relative bradycardia should only be applied to patients with a temperature >102°F (38.9°C), since differences between heart rate and temperature readings ⱕ102°F might be insufficient to discern pulse temperature abnormalities.10 Our assessment of this more febrile subgroup yielded similar results to those of the entire study population. FIGURE 1. A, Change in heart rate (HR) during acute scrub typhus infection. The upper limit of the bars represents the median febrile HR measured during acute infection with Orientia tsutsugamushi in patients with a relative bradycardia (RB) response to infection (n ⳱ 53) and patients with a normal pulse increase (NPI) (n ⳱47). The lower limit of the bars represents the median basal HR measured following recovery from infection. * P < 0.05, ** P < 0.0001 for comparison with the RB group. B, Relationship between resting HR and the pulse response to fever for RB patients (䊊) and NPI patients (䊉). 479 RELATIVE BRADYCARDIA IN SCRUB TYPHUS Although the group of patients exhibiting relative bradycardia during scrub typhus had a higher resting heart rate than did those who mounted a normal, febrile heart rate increase, the basal heart rate for a given patient was not predictive of their febrile heart rate response. The factors determining an individual’s cardiovascular response during fever are complex and not fully understood.2 During the febrile response, a decrease in systemic vascular resistance is accompanied by an increase in the heart rate aimed at maintaining/ enhancing cardiac output.4,5 In addition, pyrexia itself may induce tachycardia through thermal effects upon the sinus node.2 Cytokines elaborated during infection, such as tumor necrosis factor (TNF)-␣, interleukin-1 (⌱L-1) and IL-6, may also directly alter a patient’s hemodynamic status, independent of effects of warmth.2 In this regard, it is interesting that relative bradycardia has been found to occur during typhoid fever,7 an infection that can be accompanied by a depressed ability of peripheral blood leukocytes to release proinflammatory cytokines such as TNF-␣ and IL-1.17 However, a study assessing the relationship between cytokine levels and the heart rate response during typhoid fever has not been conducted. Whether relative bradycardia occurs more commonly in scrub typhus than in other relative bradycardia−associated infections is unclear, since differences in study design, sample size, and definition of relative bradycardia make comparison among studies difficult. Our definition of a relatively slow cardiac response to pyrexia of <10 beats/minute/°C represents the lower end of the normal febrile heart rate increase of 10−18 beats/minute/°C during infectious and noninfectious fever.1,3−6 This definition of relative bradycardia has been applied to other patient populations.7 However, the application of alternative definitions of relative bradycardia would greatly affect our results. For example, other investigators have defined relative bradycardia as a cardiac response of <10 beats/minute/°F (18 beats/minute/°C) in the setting of scrub typhus.12 The prevalence of relative bradycardia in our population increases from 53% to 92% when this cut-off value is used. Conversely, if a definition of 8.5 beats/minute/ °C as the normal cardiac response to fever is used, as reported in a small study of ill young men,18 the prevalence of relative bradycardia in our cohort would decrease to 40%. The microbial and/or host factors responsible for eliciting a relatively slow pulse response to infection are not well understood. The majority of organisms reported to be associated with relative bradycardia are intracellular pathogens. It is speculative that an occult intracellular habitat might alter neural or humoral signals regulating heart rate during infection. Orientia tsutsugamushi is atypical as a gram-negative organism in that it lacks both peptidoglycan and lipopolysaccharide.11 Whether these structural differences might alter the cardiovascular response of an infected host is unclear. In addition, the clinical significance of relative bradycardia during infection is unknown. In summary, clinically mild infection with O. tsutsugamushi is often associated with relative bradycardia. The discovery of relative bradycardia in a febrile patient at risk for scrub typhus should alert health care providers to this diagnosis. Further studies are required to understand the clinical significance of relative bradycardia in scrub typhus. The development of a standard definition of relative bradycardia may aid in the design of future investigations. Received October 2, 2002. Accepted for publication January 9, 2003. Acknowledgment: This work was presented in part at the 40th Annual Meeting of the Infectious Diseases Society of America, Chicago, October 2002. Financial support: This study was supported by the U.S. Army Research and Material Command and the Royal Thai Army. Disclaimer: The opinions or assertions contained in this report are the private views of the investigators and are not to be construed as official or reflecting the views of the U.S. Army or the Royal Thai Army. Authors’ addresses: David M. Aronoff, Divisions of Infectious Diseases and Pulmonary and Critical Care Medicine, University of Michigan Health System, 6301 MSRB III, 1150 W. Medical Center Drive, Ann Arbor, MI 48109-0642, Telephone: 734-763-9077, Fax: 734-764-4556, E-mail: [email protected]. George Watt, HIV Interaction Section, Retrovirology Department, Armed Forces Research Institute of Medical Sciences, APO AP 96546, Telephone: 66-2-644-6735, Fax: 66-2-246-8908, E-mail: [email protected]. army.mil or [email protected] REFERENCES 1. Lyon DM, 1927. The relation of pulse-rate to temperature in febrile conditions. Q J Med 20: 205–218. 2. Greisman SE, 1991. Cardiovascular alterations during fever. Mackowiak PA, ed. Fever: Basic Mechanisms and Management. New York: Raven Press, 143−165. 3. Altschule MD, Freedberg AS, McManus MJ, 1945. Circulation and respiration during an episode of chill and fever in man. J Clin Invest 24: 878–889. 4. Moser KM, Perry RB, Luchsinger PC, 1963. Cardiopulmonary consequences of pyrogen-induced hyperpyrexia in man. J Clin Invest 42: 626–633. 5. Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE, 1989. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med 321: 280–287. 6. Pernerstorfer T, Schmid R, Bieglmayer C, Eichler HG, Kapiotis S, Jilma B, 1999. Acetaminophen has greater antipyretic efficacy than aspirin in endotoxemia: a randomized, double-blind, placebo-controlled trial. Clin Pharmacol Ther 66: 51–57. 7. El-Newihi HM, Alamy ME, Reynolds TB, 1996. Salmonella hepatitis: analysis of 27 cases and comparison with acute viral hepatitis. Hepatology 24: 516–519. 8. Ostergaard L, Huniche B, Andersen PL, 1996. Relative bradycardia in infectious diseases. J Infect 33: 185–191. 9. Wittesjo B, Bjornham A, Eitrem R, 1999. Relative bradycardia in infectious diseases. J Infect 39: 246–247. 10. Cunha BA, 2000. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect 6: 633–634. 11. Amano K, Tamura A, Ohashi N, Urakami H, Kaya S, Fukushi K, 1987. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect Immun 55: 2290– 2292. 12. Machella TE, Forrester JS, 1945. Mite or scrub typhus: a clinical and laboratory study of 64 cases. Am J Med Sci 38: 38–61. 13. Sayen JJ, Pond HS, Forrester JS, Wood FC, 1946. Scrub typhus in Assam and Burma: a clinical study of 616 cases. Medicine (Baltimore) 25: 155. 14. Hazlett DR, 1970. Scrub typhus in Vietnam: experience at the 8th Field Hospital. Mil Med 135: 31–34. 15. Fang RC, Lin WP, Chao PS, Kuo NT, Chen CM, 1975. Clinical observations of scrub typhus on Penghu (the Pescadores Islands). Trop Geogr Med 27: 143–150. 16. Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D, 2000. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet 356: 1057–1061. 17. House D, Chinh NT, Hien TT, Parry CP, Ly NT, Diep TS, Wain J, Dunstan S, White NJ, Dougan G, Farrar JJ, 2002. Cytokine release by lipopolysaccharide-stimulated whole blood from patients with typhoid fever. J Infect Dis 186: 240–245. 18. Karjalainen J, Viitasalo M, 1986. Fever and cardiac rhythm. Arch Intern Med 146: 1169–1171.