* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Relativistic molecular structure calculations for the detection of CP
Eigenstate thermalization hypothesis wikipedia , lookup
Elementary particle wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Quantum chromodynamics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Canonical quantization wikipedia , lookup
Spin (physics) wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Standard Model wikipedia , lookup
Renormalization wikipedia , lookup
Photon polarization wikipedia , lookup
History of quantum field theory wikipedia , lookup
Scalar field theory wikipedia , lookup
Nuclear structure wikipedia , lookup
Mathematical formulation of the Standard Model wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Renormalization group wikipedia , lookup
Electron scattering wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
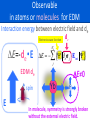
Symposium: New Generation Quantum Theory -Particle Physics, Cosmology, and ChemistryKyoto University, Yoshida Campus 7-9 March 2016 TMU Relativistic molecular electronic structure calculations for the detection of CP violation Be a chemist now! Minori Abe 阿部穣里 Tokyo Metropolitan University [email protected] Outline 1. CP symmetry and electron’s electric dipole moment (eEDM) 2. eEDM operator in molecule and effective electric field (Eeff) 3. Eeff in YbF, HgX and BiO molecules: Relativistic CCSD and CASPT2 results Outline 1. CP symmetry and electron’s electric dipole moment (eEDM) 2. eEDM operator in molecule and effective electric field (Eeff) 3. Eeff in YbF, HgX and BiO molecules: Relativistic CCSD and CASPT2 results A mystery in Physics Why anti-particles almost die out in our universe, even though the same number of particles and anti-particles are created in Big-Bang ? Disappearance of anti-particles particles and anti-particles obey different laws Sakharov’s three necessity conditions 1.Charge symmetry and Charge-Parity (CP) symmetry violation 2.Baryon number violation 3.Interactions out of thermal equilibrium CP(Charge-Parity) symmetry violation Charge conjugate +q Parity inversion -q The standard model predicts CP violation, but it is very weak and cannot explain the too small amount of anti-particles in the present universe. We need a new theory and experimental evidences which explains much larger CP violation. Electric dipole moment (EDM) of an elementary particle EDM d EDM -d Time reversal spin (t -t) (p-p) (rr) L-L Assume that EDM is parallel to spin axis spin dd EDM becomes antiparallel to spin axis Non-zero value of EDM = T symmetry violation = CP symmetry violation (under CPT theorem) Upper limit of electron EDM observed in atoms and molecules Why upper limit because the errors are larger than the observables at present. Molecules are hot now! If an absolute value of EDM is determined, it will be a great hint for the models beyond the standard theory. WC, HfF+, BiO, FrSr,… YbF ThO Outline 1. CP symmetry and electron’s electric dipole moment (eEDM) 2. eEDM operator in molecule and effective electric field (Eeff) 3. Eeff in YbF, HgX and BiO molecules: Relativistic CCSD and CASPT2 results Observable in atoms or molecules for EDM Interaction energy between electric field and de Electronic wave function E=-de・E E spin matrices Ne E d e σ j Eint j E=0 EDM de spin de Yb When spherically symmetric orbital Internal electric field Eint Observable in atoms or molecules for EDM Interaction energy between electric field and de Electronic wave function E=-de・E Ne E de σ j Eint j E≠0 EDM de spin E de Yb when symmetry is broken by Eext Eext Observable in atoms or molecules for EDM Interaction energy between electric field and de Electronic wave function E=-de・E de Ne E de σ j Eint j E≠0 EDM de spin E Yb F In molecule, symmetry is strongly broken without the external electric field. Problem of EDM operator Ne Effective electric field E d e σ j Eint d e Eeff j σ j Eint σ j , V (r ) σ j , Hˆ 0 Unperturbed 2-component nonrelativistic Schrödinger eq. 1 2 ˆ H 0 i V 2 i Ĥ 0 E0 Ne Eeff σ , Hˆ 0 0 j We cannot measure E. (Schiff’s theorem) Relativistic EDM operator Ne E d e σ j Eint d e Eeff j 2 ˆ σ j Eint σ j , H 0 2ic 5p j Unperturbed 4-component relativistic Schrödinger eq. Ne Hˆ 0 c pi βmc 2 V Ĥ 0 E0 i Ne Eeff 2ic 5p 2 0 j Relativity is important to observe EDM. Relativistic quantum chemistry is necessary Ne H eEDM d e σ j Eint E Hˆ eEDM j N nuc ZA 2 ˆ H 0 c pi βmc i A ri R A N elec L L S S 4-cmp spinor Nelec 1 i j ri r j Dirac-Coulomb Hamiltonian Eeff 1 d e Hˆ eEDM We cannot measure Eeff experimentally and need to calculate. Collaborations of three fields are very important! Particle Physics (Theory) d e i Eint i Atomic, Molecular, and Optical Physics (Experiment) Relativistic Quantum Chemistry to calculate Eeff Effective one-body operator Ne H eEDM d e σ j Eint E Hˆ eEDM j 2 σ Eint σ , Ĥ 0 2ic 5p Eeff 1 d e Hˆ eEDM Ne 2ic 5p 2 j Effective one-body operator The expectation value of the effective operator is exactly same to the original one when the wave function is exact solution of Ĥ 0 . One electron orbital form 2ic i4 cmp 5 p 2 i4 cmp 2ic iL p 2 iiS iiS p 2 iL 2ic i iL p 2 iS i iL p 2 iS 022 5 122 4c Re iL p 2 iS 122 022 Coupling of large and small components N elec Eeff ROHF 4c Re p L i 2 S i i At the Kramers restricted ROHF level, the p2 value of SOMO is only remained. 4c Re p singly occupied molecular orbital (SOMO) L SOMO 2 S SOMO Large and small component of atomic spinor (basis sets) Large component Small component Yanai et al. JCP, 114, 6526, 2001. Differentiation and angular moment c σ p L L σ p 2 2mc V 2mc c L σ p S V S p L 2 S L σ p σ p S Differentiation of s p Differentiation of p … s and d Kinetic balance condition V L σ p L c s p p Mixture of s and p spinor in SOMO is important. (s can be close to the nucleus having large Eint.) Larger Eeff is better for experiment Larger Eeff provides larger value of E in experiment. When Eeff can be large? • Paramagnetic (otherwise zero) • Molecule with heavy element nucleus X because of its large Eint • SOMO is important! SOMO cs s , X c p p , X SOMO distributing to the heavy nucleus X SOMO with parity mixing Mixing of s and p-spinor may be the best. Diatomic molecule with large Eeff Q. Choose two atoms to create diatomic molecule with large Eeff Ask Mr. Sunaga For example of XF molecules SOMO mainly consists of s. Virtual p will be mixed in SOMO. X: (ns)2 F: (2p)5 X+: (ns)1 F-: (2p)6 Outline 1. CP symmetry and electron’s electric dipole moment (eEDM) 2. eEDM operator in molecule and effective electric field (Eeff) 3. Eeff in YbF, HgX and BiO molecules: Relativistic CCSD and CASPT2 results Previous theoretical works (Eeff) Atomic EDM: Dirac-Coulomb + CCSD, etc ・Accurate calculations are reported for both relativity and electron correlation. Molecular EDM (PbO,TlF,ThO,YbF…) : (One or two-component ) GRECP+RCCSD, GRECP+SOCI (Four-component ) RASCI, GASCI ・Only a few works are reported, enough accurate in relativity, electron correlation, and basis set size. Our motivation Calculated Eeff cannot be compared with any experiments We should calculate Eeff as precise as possible with the techniques of quantum chemistry and available computational resource! Relativity (Dirac-Coulomb) Basis sets (Dyall QZ) Electron correlation (CCSD, CASPT2) YbF molecule SOMO mainly consists of s. Virtual p will be mixed in SOMO. X: (ns)2 F: (2p)5 X+: (ns)1 F-: (2p)6 YbF molecule Experimentally reported Simple electronic structure Bench mark of calculations Couple cluster singles and doubles (CCSD) b a Virtual orbitals a j Occupied orbitals i i ΦHF HF determinant Φ𝑖𝑎 =𝐸𝑖𝑎 ΦHF Singly excited det. 𝑇1 = 𝑡1,𝑖𝑎 𝐸𝑖𝑎 𝑖,𝑎 𝑎𝑏 𝑎𝑏 Φ𝑖𝑗 = 𝐸𝑖𝑗 ΦHF Doubly excited det. 𝑇2 = 𝑡2,𝑖𝑗𝑎𝑏 𝐸𝑖𝑗𝑎𝑏 𝑖,𝑗,𝑎,𝑏 ΦCCSD = exp(𝑇1 + 𝑇2 ) ΦHF An effective method when the HF determinant is the only dominant determinant. Computational details for YbF Phys. Rev. A 90, 022501 (2014) Wave function: Dirac-Fock, Dirac-CCSD Program : UTChem + DIRAC08 Basis sets: Dyall’s DZ, TZ, and QZ for Yb + Watanabe’s basis for F + Sapporo (DK3) polarization in uncontracted form We approximate the expectation value with CCSD wave function as follows. ˆ ˆ ˆ ˆ ˆ O HF 1 T1 T2 ON 1 T1 T2 HF ˆ HF HF O C Measurable properties related with Eeff • Hyperfine coupling constant (parallel) A//: Interaction between electron spin and Yb nuclear spin • Molecular dipole moment PDM: s-p hybridization QZ 79e-CCSD(293) Experiments Error from experiments Eeff (GV/cm) A// (MHz) PDM (D) 23.1 7913 7424 7% 3.60 3.91 8% - Eeff in HgX (X=F, Cl, Br, I) Basis set Molecule type Mr. Prasannaa HgF HgCl HgBr HgI DZ DZ DZ DZ Method Eeff (GV/cm) CCSD CCSD CCSD CCSD 115.42 113.56 109.29 109.30 HgX has much larger Eeff than YbF (23.1 GV/cm). “Mercury Monohalides: Suitability for Electron Electric Dipole Moment Searches” V. S. Prasannaa, A. C. Vutha, M. Abe, and B. P. Das Phys. Rev. Lett. 114, 183001, (2015)