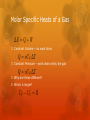* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermodynamics: Kinetic Theory
Survey
Document related concepts
Relativistic quantum mechanics wikipedia , lookup
Path integral formulation wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Matter wave wikipedia , lookup
Topological quantum field theory wikipedia , lookup
EPR paradox wikipedia , lookup
Scalar field theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
Renormalization wikipedia , lookup
Canonical quantization wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Atomic theory wikipedia , lookup
History of quantum field theory wikipedia , lookup
Renormalization group wikipedia , lookup
Transcript
Thermodynamics: Kinetic Theory From Warmup The chapter just seemed like a ton of equations. I was confused about what its point was. Can you explain that? Writing out 8-2A helped, but I still barely understand anything after 21-2. The amount of equations that are being juggled around and the various equalities and substitutions being made are more than I can follow without a clearer way to walk through it all. This chapter had, like, 44 equations. Do we have to know them all? You are physicists. You should have a working knowledge of the entire universe and everything it contains…. I usually go over the whole derivation in class (“reading math” is an important skill), but today we are pressed for time. So let me give you the highlights…. My slides (with more details than we will cover) are on the course website. Organization of Scientific Theories Fields of science are organized hierarchically Simple theories of complex processes help us understand the world. These simple theories operate as though the complexity did not exist! Newtonian Mechanics vs. Quantum Mechanics Quantum Mechanics vs. string theory Thermodynamics vs. Statistical Mechanics Do you really need to know how all the molecules in a gas are moving to understand how the gas will behave? But isn’t the behavior of the gas determined by the motion of the molecules? Emergence and complexity Fluids Solids Chemistry Biology Macroscopic Emergence Complexity Atoms Microscopic First principles Microscopic Model to Macroscopic theory d Microscopic Model to Macroscopic theory d Microscopic Model to Macroscopic theory (One particle) (Whole gas) d On long time scales, the many small impulses behave like a large, continuous force Microscopic Model to Macroscopic theory Microscopic Model to Macroscopic theory Microscopic Model to Macroscopic theory Success! A microscopic model relating to a macroscopic observable! The Big Idea: If the molecular motion determines the behavior of the gas, why do I not need to know all the microscopic details to understand the gas? Only the average energy matters. I can distribute that energy in different ways and the gas will look identical to me on the macroscopic scale. Preview: Entropy (next section), Physics 360 Velocity, energy, and mass of gases In air, the molecular mass of oxygen molecules is 32 g/mol. The molecular mass of nitrogen molecules is 28 g/mol. Which molecules are traveling faster on average? A) Oxygen B) Nitrogen C) Same Speed Emergence Temperature and Pressure don’t “look” like energy and collisions at first. They obey different laws Where is momentum conservation in PV = NkT? On large, macroscopic scales, new physics emerges, that is fundamentally different from the microscopic physics. Macroscopic physical laws appear to exist almost independently of the microscopic laws. Thermodynamics / Statistical Mechanics Knowing the microscopic laws does not mean you understand the macroscopic laws. Understanding the relationship between the microscopic/macroscopic is one of the major open problems in fundamental science today. Microscopic/Macroscopic Theories Many theories have a macroscoipc/microscopic relationship: Thermodynamics / Statistical Mechanics Newtonian Mechanics / Quantum Mechanics Particle Physics / String Theory Climate / Weather Macroeconomics / Microeconomics Biology / Chemistry Material Science / Quantum Mechanics Molar specific heat: If I add some energy to a gas, how much does its temperature change? (Let’s assume there is no work, i.e. Volume = constant) This equation is true for molecules with no internal structure. If molecules can vibrate or rotate, they can store more energy. Equipartition Theorem: Each degree of freedom makes an equal contribution the molar specific heat. In reality the situation is a bit more complicated…. Quantum mechanics Gases can also do work Hints of Quantum Physics Clicker Quiz What is the molar specific heat of an ideal gas of diatomic molecules at room temperature? A) 3/2 R B) 4/2 R C) 5/2 R D) 6/2 R E) 7/2 R Count the degrees of freedom: Translational motion: 3 Rotational motion: 2 (why not 3?) Vibrational motion: 0 (why 0?) Molar Specific Heats of a Gas Constant Volume – no work done Constant Pressure – work done on/by the gas Why are these different? Which is larger?