* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Compare the origin and subsequent evolution of mitochondria and
Survey
Document related concepts
Ridge (biology) wikipedia , lookup
Genomic imprinting wikipedia , lookup
Endomembrane system wikipedia , lookup
Gene expression wikipedia , lookup
Community fingerprinting wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Gene expression profiling wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Non-coding DNA wikipedia , lookup
Gene regulatory network wikipedia , lookup
List of types of proteins wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Transcript
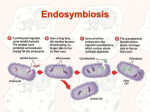
Jessica Griffiths Compare the origin and subsequent evolution of mitochondria and chloroplasts. Mitochondria and chloroplasts, the ‘powerhouses’ of eukaryotic cells, are absolutely fundamental to understanding the sequence of events underpinning eukaryotic evolution. The endosymbiosis theory proposes that chloroplasts and mitochondria found in present-day eukaryotic cells evolved from bacteria endocytosed by ancient eukaryotes. Molecular evidence suggests that mitochondria evolved from endocytosed proteobacteria, whilst chloroplasts originate from an oxygen-producing photosynthetic organism resembling a cyanobacterium. The symbiotic event giving rise to mitochondria is thought to have occurred over one and a half billion years ago, following a substantial increase in atmospheric oxygen levels. The chloroplast appears to have been similarly derived around one billion years ago, after mitochondria, since all eukaryotic cells contain mitochondria, but not chloroplasts. This is serial endosymbiosis: an ancient eukaryote engulfed the mitochondrion ancestor, and some descendants of it then engulfed the chloroplast ancestor, giving rise to a cell containing both chloroplasts and mitochondria. The mitochondria and chloroplasts were endocytosed by the ancient eukaryote either as food, or an internal parasite. They managed to escape the phagocytic vacuole within which they were contained. The double lipid-bilayer membranes that surround all chloroplasts correspond to the gram-negative cell walls of the ancestral cyanobacterium, not the host’s phagosomal membrane, which was likely lost throughout evolution. Ancient eukaryotes were able to subvert the oxidative phosphorylation systems of the endocytosed bacteria in the endosymbiosis giving rise to mitochondria. Similarly, the endosymbiotic relationship giving rise to chloroplasts allowed the ancient eukaryotes to harness the photophosphorylation mechanism employed by the ancestral cyanobacteria. The resulting increase in ATP production in both cases made the symbiosis energetically favourable for the host eukaryotes. In return, the endocytosed bacteria were provided with protection, nutrients and a stable environment. These relationships were therefore mutually-beneficial, and so were selected for in the course of evolution. Studies have revealed that mitochondria and chloroplasts contain their own genetic systems, which are separate and distinct from the eukaryotic nuclear genome. Mitochondria and chloroplasts divide by binary fission. Before each division, their DNA is replicated, and the genes encoded are transcribed within the organelle, and translated on the organelle ribosomes. Mitochondrial and chloroplast DNA is circular and similar in size and structure to bacterial plasmids. Their 70S ribosomes and ribosomal RNA also more closely resemble those in bacteria than those in eukaryotes. These similarities in the biochemistry and molecular biology of mitochondria and plastids in modernday eukaryotes and ancestral bacteria provide further evidence for the endosymbiosis theory. There are, however, many significant differences that exist between the mitochondria and chloroplasts, and the ancestral bacteria from which they were obtained. These differences can provide insight into the evolution of modern eukaryotes. Sequencing of the mitochondrial genome has revealed that the coding capacity of the modern day mitochondrial genome is much smaller than their closest eubacterial relatives. Sequenced plastid genomes encode from 20 to 200 proteins, and mitochondrial genomes encode anywhere from 3 to 67 proteins. The closest relatives of mitochondrial and chloroplast genomes are alpha-proteobacteria and cyanobacteria respectively. The modern alpha-proteobacteria Mesorhizobium loti contains 7 Mb of DNA encoding over 6,700 proteins, whilst the cyanobacterium Nostoc PCC 7,120 has a 6.4 Mb genome, which encodes approximately 5,400 proteins. Comparison of the genome sizes of these bacterial ancestors and mitochondria and chloroplasts illustrates the magnitude of organelle genome reduction. [2] Jessica Griffiths During eukaryote evolution, there therefore must have been an extensive transfer of genes from the organelles to the nuclear genome. This is consistent with increased dependence on the eukaryotic host following endosymbiosis. However, successful transfers of this type are rare, due to the fact that the organelle gene needs to change to become a functional nuclear gene – it must adapt to the nuclear and cytoplasmic and nuclear transcription and translation requirements, and must obtain a signal sequence so that the encoded protein can be transported back to the chloroplast or mitochondria following synthesis, with the help of transit peptides and protein-import machinery. Nevertheless, it is possible; through experimentation, Guang et al. made a minimum estimate that one pollen grain in 16,000 in tobacco undergoes a chloroplast-to-nucleus transfer. This demonstrates that endosymbiotic gene transfer is possible, and that such gene transfers continue to occur today. Endosymbiotic gene transfer is advantageous for a number of reasons. The nucleus is a sexual population whereas the chloroplasts and mitochondria are not, because of uniparental inheritance. In asexual reproduction, genomes are inherited as indivisible blocks, meaning that irreversible deleterious mutations are inherited by future generations, and so eventually accumulate. This genetic load eventually becomes so great that the population goes extinct. However, in sexual populations, genetic recombination ensures that the genomes of the progeny differ from the parental genomes, allowing progeny genomes with fewer mutations to be generated. Transfer of genes from chloroplasts and mitochondria to the sexually-recombining gene pool of the nucleus is therefore favourable. Another benefit of endosymbiotic gene transfer is that the nucleus reduces the exposure of DNA to reactive oxygen species generated by oxidative phosphorylation in mitochondria and photosynthesis in chloroplasts. This helps to protect the DNA from damage. DNA transfer from the organelles to the nucleus also helps to ensure that the division of the cell and organelles (which reproduce by binary fission) are synchronous. Despite these advantages, some genes are still retained within mitochondria and chloroplasts. This enables the control of the expression of genes encoding components of their electron transport chain, allowing them to synthesise these components when needed. This helps to maintain redox balance, thereby decreasing production of toxic reactive oxygen species. Local transcription and translation of organelle genes allows for a quicker and more direct response. It also allows organelles to produce personalised responses specific to them – proteins encoded by nuclear genes would be delivered to all mitochondria or chloroplasts. Another plausible explanation for the retention of certain genes in mitochondria and chloroplasts is the existence of a limited transfer window – the possibility of gene transfer may have ended before everything could be transferred. DNA sequencing also shows that deletion of certain genes within the genomes occurred. For example, complex I genes in Saccharomyces cerevisciae have been lost, leading to the loss of the first coupling site in the yeast’s electron-transport chain. [1] In some of these cases, the deleted gene’s function may be carried out by unrelated nuclear genes instead. Despite these changes in the mitochondrial and chloroplast genomes, in a large number of cases, the protein-coding genes are in the same order as their bacterial homologues, providing evidence for a single, endosymbiotic origin. Strong evidence for a single endosymbiotic origin of mitochondria is also provided by the presence of hydrogenosomes and mitosomes in amitochondriate organisms. The presence of N-terminal targeting peptides – conserved protein import machinery - in these organelles shows that they are in fact mitochondrial remnants. All known lineages of eukaryotes containing mitosomes or hydrogenosomes also branch as sisters to mitochondrion-bearing lineages. It is therefore widely accepted that amitochondriate organisms lost their mitochondria during the course of evolution, as opposed to forming a monophyletic group before the establishment of mitochondria. Jessica Griffiths . It has been debated and disputed whether chloroplasts also originated from a single endosymbiotic event, or many independent engulfments in different eukaryotic lineages. It is most widely accepted that they share a single ancestor resembling a cyanobacterium (with one exception of one species Paulinella chromatophora - which arose from an independent primary endosymbiosis separate from land plants and green-algae). Despite this single eukaryotic origin, chloroplasts are found in an extremely large variety of organisms – this is a consequence of many secondary and tertiary endosymbiotic events. This can be seen in cryptophyte algae, which are surrounded by four membranes – the two additional membranes corresponding to the plasma membrane of the engulfed cell, and the phagosomal membrane of the host cell. The remnant nucleus – the nucleomorph – from an intermediate photosynthetic eukaryote, is present between the two of the plastid membranes. The topography of plastid membranes is an evolutionary signature of secondary endosymbiosis. The endosymbiotic relationships giving rise to chloroplasts and mitochondria are some of the most important organisational events in the history of life. The origin and subsequent evolution of mitochondria and chloroplasts demonstrates the importance of co-operation in evolutionary change. They have far-reaching evolutionary implications, and are absolutely essential to the existence of all higher life-forms on the planet Earth. Bibliography: 1) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC138944/ 2) http://www.nature.com/scitable/content/endosymbiotic-gene-transfer-organelle-genomesforge-eukaryotic-13997492 “Endosymbiotic Gene Transfer: Organelle Genomes Forge Eukaryotic Chromosomes” by Jeremy N. Timmis, Michael A. Ayliffe, Chun Y. Huang and William Martin