* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heat
Vapor-compression refrigeration wikipedia , lookup
Hypothermia wikipedia , lookup
Radiator (engine cooling) wikipedia , lookup
Thermal comfort wikipedia , lookup
Underfloor heating wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Thermal conductivity wikipedia , lookup
Passive solar building design wikipedia , lookup
Thermoregulation wikipedia , lookup
Insulated glazing wikipedia , lookup
Solar water heating wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat equation wikipedia , lookup
Dynamic insulation wikipedia , lookup
Cogeneration wikipedia , lookup
Intercooler wikipedia , lookup
Building insulation materials wikipedia , lookup
Solar air conditioning wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
R-value (insulation) wikipedia , lookup
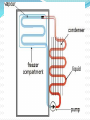
Introduction Introduction Energy Transfer – the transfer of energy from one body to another Chapter 5: Heat and Heat Transfer Introduction Snow ball activity! On looseleaf: What do you already know about heat? What is heat? How does heat transfer from one object to another? Why is controlling heat transfer important for engines and technological devices? Why is controlling heat transfer important for your own survival? Introduction Terminology Heat Energy vs. Thermal Energy These two terms are essentially the same thing. They can be used interchangeably. Introduction What causes heat? Where does it come from and why? For over a century, scientists hotly debated the answers to these questions as they worked to develop modern heat theory. Introduction In a pre-match society, starting a fire was not the simple task it is today. In many early societies, people who could start fires easily were admired, often revered. If it was your job to keep the fire going, letting it go out was considered a grievous wrong. 5.1 The Nature of Heat Friction Theory when two surfaces are rubbed together, the parts that touch resist movement This resistance is friction Count Rumford used observations about friction to change the way scientists look at heat 5.1 The Nature of Heat Benjamin Thomson was an engineer and scientist who lived in the Thirteen Colonies at the time of the American Revolution. Because he stayed loyal to Britain, many Americans considered him a traitor. British loyalists, however, considered him a hero. Thomson was given the title of Count Rumford to reward him for his loyalty to Britain. 5.1 The Nature of Heat Rumford was an engineer/scientist who was hired to manufacture cannons in Munich, Germany. During this project, one worker carelessly touched a rod being used to bore a hold through a piece of metal. His hand was seriously burned. In the late 1700s, Count Rumford observed that heat was created when metal cut metal. This heat results from friction! 5.1 The Nature of Heat Have you ever watched popcorn popping in an air popper? The random, dancing motion of the kernels is easy to observe. But what causes this motion? 5.1 The Nature of Heat A similar type of motion is occurring in a glass of water. 5.1 The Nature of Heat Robert Brown – the first to realize this similarity During the 1800s he was using a microscope to observe pollen grains in a drop of water. He noticed that although the microscope was quite still, the pollen grains bounced around. When he increased the temperature of the water, the motion increased. This motion has become known as Brownian Motion http://www.microscopy-uk.org.uk/dww/home/hombrown.htm 5.1 The Nature of Heat Making the observation was easy. But how can we explain it? 5.1 The Nature of Heat At first, Brown thought that the pollen grains were alive. Later he reasoned that water must be composed of tiny unseen particles. These particles are in constant, vibrating motion. The motion of the pollen grains must be caused by collisions between the pollen grains and the other unseen particles. (Brown was unsure of what the particles were) Later, his evidence helped develop the kinetic molecular theory. 5.1 The Nature of Heat The concept of heat is commonplace; what causes heat is fairly abstract. Technically, heat is defined as the transfer of energy from one substance to another and is identified by a difference in temperature. An object does not possess heat. Rather, an object possesses thermal energy and can lose that energy in the process of heat loss. 5.1 The Nature of Heat It is a common misconception to think of heat as a thing rather than a process. This probably stems from the original caloric definition of heat. Scientists thought that something measurable left an object when it got colder. Recall that Rumford was able to gain a scientific understanding of heat through observations of friction. 5.1 The Nature of Heat Before Rumford, scientists thought heat was a fluid they called caloric. Rumford did not replace the caloric theory. Rather, he demonstrated an inconsistency in the theory. Two later British scientists — Sir Humphry Davy and James Prescott Joule took Rumford’s ideas to the next step. 5.1 The Nature of Heat Davy, an English chemist, designed demonstrations to disprove the caloric theory. He rubbed ice and other solids with low melting points together to show that they would melt with heat from friction. 5.1 The Nature of Heat Joule determined the mechanical equivalent of heat by measuring the change in temperature produced by friction. Working in imperial measure, he found that, on average, a weight of 772 pounds falling through a distance of one foot would raise the temperature of one pound of water by 1°F. In addition, Joule’s investigations showed that heat is produced by motion, contradicting the caloric theory. 5.1 The Nature of Heat We can relate this to the calorie, which is related to food energy. The calorie is a unit of energy. One calorie is the amount of energy needed to raise 1 gram of water by 1°C. Other units of energy are the joule — named after James Prescott Joule and the British Thermal Unit (BTU). The BTU is used in Canada to rate thermal output of stoves, ovens, and barbecues. 5.1 The Nature of Heat Mysterious Motion P. 84 5.1 The Nature of Heat 5.1 The Nature of Heat Practice! Check Your Understanding p. 85 5.2 Heat and Temperature The modern theory of heat began with Robert Brown He was the first to suggest that the energy that came with heat – thermal energy – was related to the motion of unseen particles of a substance. But, what are these unseen particles? What causes the motion? 5.2 Heat and Temperature Recall the Particle Theory of Matter: All matter is composed of tiny, unseen particles These unseen particles are in constant, random motion. 5.2 Heat and Temperature Kinetic – means movement Kinetic Art – art that moves (i.e. Mobiles) Kinetic Energy – a form of energy associated with motion It is a measure of the amount of motion particles have. We can use kinetic energy to explain the difference between heat and temperature 5.2 Heat and Temperature A molecule can have three different forms of movement: Vibrational — molecules “vibrate” back and forth Rotational — molecules spin and rotate Translational — molecules bump and move around 5.2 Heat and Temperature The motion of particles can be compared to bumper cars! 5.2 Heat and Temperature Like bumper cars, atoms and molecules collide with each other at different speeds. All particles have different kinetic energies. 5.2 Heat and Temperature So what is the difference between heat and temperature? Temperature – the average of ALL kinetic energies of all particles in an object Heat – the sum of all kinetic energies of all particles in an object 5.2 Heat and Temperature Example: 25 mL Temperature: 30°C Less kinetic energy 100 mL Temperature: 30°C More kinetic energy 5.2 Heat and Temperature Because temperature is a measure of how fast molecules are, and because molecules slow as they get colder, the coldest possible temperature is finite! Particles truly stop moving at absolute zero. Absolute zero is -273.15°C The lowest artificial temperature achieved to date is 0.003°K. The highest is estimated at 100 000 000°K and was from a nuclear blast. 5.2 Heat and Temperature There are more temperature scales than Celsius and Fahrenheit. The Kelvin scale (K) starts at absolute zero and rises in degrees equal in magnitude to Celsius degrees. Other scales include the Rankine scale and the International Temperature Scale. 5.2 Heat and Temperature Practice! Check Your Understanding p. 87 5.3 Transfer of Heat There have been many predictions about when Earth will end. Fear of Y2K computer problems brought excitement to New Year’s Eve 2000. As we study heat transfer, we will learn about another proposed end to the universe 5.3 Transfer of Heat Forms of Heat Transfer Heat flows from hot to cold. The flow continues until both objects are at the same temperature. But what is really happening? How does thermal energy transfer from one object to another? 5.3 Transfer of Heat Conduction Molecules placed on a hot burner will vibrate quickly. They have more kinetic energy than the molecules in a cooler location (a cool pot). Contact between the pot and the burner causes the molecules of the hot burner to collide with the slower molecules of the cool pot. These collisions result in a transfer of kinetic energy. 5.3 Transfer of Heat The molecules of the cooler pot start to vibrate faster, gaining kinetic energy. The molecules of the hot burner vibrate slower, losing kinetic energy. This transfer of heat by contact is called conduction. 5.3 Transfer of Heat Thermal conductivity and electrical conductivity are related. Substances that conduct electricity will also tend to be good thermal conductors. Metal is a good example of this. Conversely, glass and wood are poor thermal and poor electrical conductors. 5.3 Transfer of Heat Convection If you hold your hand above a hot burner of a stove, you will feel a warm current of air. Why? Heat is transferred, by conduction, from the hot burner to the air molecules touching the burner. These air molecules gain kinetic energy, vibrate faster, and get farther apart. 5.3 Transfer of Heat The warmer molecules are farther apart than the cool air molecules, so the warm air is less dense than the cool air around it. This causes the warm air to rise, creating the warm current that we feel. Cool, denser air rushes to take the place of the warm air. 5.3 Transfer of Heat Because of continuous air flow, all the air in the room will become warmer. This transfer of heat by movement is called convection. 5.3 Transfer of Heat Radiation Picturing a hand that is close to the side of a burner, but not above the burner can help us explain radiation. The front of the hand is not being heated by conduction or convection. There is no contact with the burner and convection currents of warm air would rise away from the hand. 5.3 Transfer of Heat The front the hand is being heated by radiation. Radiation is produced by vibrating electrons, which are tiny particles present in all atoms. This vibration makes a wave called an electromagnetic wave or infrared radiation wave. These waves are similar to the waves your hand can create when it vibrates in calm water. Waves or ripples run away from your moving hand. 5.3 Transfer of Heat Infrared radiation waves travel from the burner. They strike the hand and transfer heat energy to the molecules in the hand. This causes molecules in the hand to vibrate faster. 5.3 Transfer of Heat On a sunny day, most of the heat that you feel is the result of infrared radiation from the Sun. Infrared radiation is one form of electromagnetic radiation. Other forms include ultraviolet radiation, radio waves, visible light waves, and X rays. Electromagnetic radiation travels in waves. Each type of electromagnetic radiation has a specific wavelength and moves through the vacuum of space. 5.3 Transfer of Heat 5.3 Transfer of Heat Did You Know? Hot objects warm cool ones until their temperatures are the same. Some people believe this will happen to the universe. According to the theory, the temperature of the entire universe will eventually be the same. When this happens, heat will no longer transfer. Without a source of energy, life will be impossible! This prediction is called the “Heat Death of the Universe.” 5.3 Transfer of Heat Practice! Check Your Understanding p. 91 5.4 Heat Transfer in Nature Convection causes many weather phenomena, such as winds. Many cottages are on the shores of lakes, where convection currents keep the cottages cool in the daytime and warmer at night. In the evening, the air over the water cools more slowly than the air over the land. Warm air from over the water rises and moves toward the land, keeping it warm. During the day, cool air from the lake moves towards the warm land. 5.4 Heat Transfer in Nature Sea and Land Breezes Recall: warm air rises, and cool air sinks The circular movement that results is called convection. All winds start with convection currents. 5.4 Heat Transfer in Nature Land and sea breezes are convection currents of air that occur near a shoreline. They are both created by differences in temperature near the surface of the Earth. 5.4 Heat Transfer in Nature On a sunny day, radiant heat from the Sun strikes Earth’s surface. Heat is absorbed by land and water. But land and water heat at different rates. Land heats quickly, but also cools quickly. Water heats slowly and takes longer to cool. 5.4 Heat Transfer in Nature 5.4 Heat Transfer in Nature How Oceans Help to Moderate Climates Oceans are capable of storing large amounts of thermal energy. This prevents the area around them from having extreme temperature changes. Oceans moderate the climate of land areas near them. This means they prevent the area from becoming too hot or too cold. But how??? 5.4 Heat Transfer in Nature In cool weather, an ocean can release great amounts of heat without cooling much itself. Even during very hot or very cold days, and as seasons change, the temperature of oceans remains constant. As the sun warms the air above the ocean, heat flows from the air into the water, cooling the air. When the temperature of the air above the ocean cools, heat flows from ocean to air and warms the air. 5.4 Heat Transfer in Nature We could say that oceans prevent land near them from getting very warm or very cold. Because of this, coastal cities (like Vancouver) have moderate climates. 5.4 Heat Transfer in Nature 5.4 Heat Transfer in Nature Practice! Check Your Understanding p. 97 Heat Transfer Review (worksheet) Midpoint Review (worksheet) 5.5 Heat Transfer and Technologies Many household technologies either transfer or prevent the transfer of heat through conduction, convection or radiation. That is why metal cooking pots have hard plastic or wooden handles. What other techniques are used to handle hot food? 5.5 Heat Transfer and Technologies Stoves Most cooks want to control how food is heated. Consider the following: heating soup on a stove top. The pot is heated by conduction through contact with the burner. The soup at the bottom of the pot heats up first through conduction. 5.5 Heat Transfer and Technologies Heated soup rises to the top, because it is less dense. Colder soup near the top of the pot flows to the bottom because it is more dense. This causes a circular motion (aka convection currents) and allows the entire pot of soup to heat to a uniform temperature. 5.5 Heat Transfer and Technologies Ovens When an oven is turned on, convection currents move heat inside the oven. Air at the bottom of the oven is heated by burning gas or an electric element. The heated air becomes less dense and rises to the top of the oven. The cooler air sinks to the bottom. 5.5 Heat Transfer and Technologies The hot air heats the oven walls. These walls then radiate heat in all directions. Food in an oven then becomes cooked by both convection and radiation. There is also conduction if a baking pan is used. 5.5 Heat Transfer and Technologies Did you know? Light-coloured surfaces reflect more heat than dark surfaces. This is why people in hot countries often wear lightcoloured clothing. 5.5 Heat Transfer and Technologies Getting Rid of the Heat Combustion of fuel inside an engine produces a large quantity of thermal energy. If this energy were not removed, the engine would overheat and be damaged. How is it protected? 5.5 Heat Transfer and Technologies The engine’s cooling system contains a liquid coolant (most likely antifreeze). The coolant is pumped through the engine block to the radiator. A radiator is a honeycomb made of a metal alloy. The metal alloy is a good conductor. Heat from the coolant is conducted through this alloy to air. 5.5 Heat Transfer and Technologies Either a fan or the motion of the vehicle forces this air through the radiator. Heat is transferred to the air that rushes through the radiator. These three techniques use conduction to protect engines from heat damage. 5.5 Heat Transfer and Technologies Keeping it Cool When you put a little water on the back of your hand, your hand becomes cool. That is because thermal energy transfers from your hand to the water through conduction. Water absorbs heat from your hand and evaporates. Evaporation removes thermal energy as the water molecules leave the water droplets and move into the air. Your hand feels cooler. 5.5 Heat Transfer and Technologies This form of cooling is usually called cooling by evaporation. The evaporation caused the cooling, but the cooling action started with conduction of heat away from your hand. 5.5 Heat Transfer and Technologies Heat Transfer in a Refrigerator A. A fluid called a coolant circulates through the pipes. B. Heat from the food transfers to the cooler air surrounding it. Thermal energy then transfers from the air to the coolant. C. The coolant evaporates as it gets warmer. It is pumped to the compressor. D. When it reaches the compressor, pressure is applied to change it back into a liquid. E. The liquid coolant is pumped to these coils. Thermal energy is released into the room. The cycle starts again. 5.5 Heat Transfer and Technologies Air conditioners work in a similar manner. In this case, the back of the unit is outside the room or house. To keep the inside of the room cool, heat is dispersed to the outside air. Although useful, coolant technology has an environmental cost. For the past 50 years, the liquid coolant used in refrigerators and air conditioners has been liquid chlorofluorocarbons, or CFCs. CFCs are responsible for atmospheric ozone depletion. As of January 2000, worldwide production of the most dangerous CFCs was to be replaced by alternative liquid coolants. Research is underway to produce environmentally safer but effective coolants. 5.5 Heat Transfer and Technologies Practice! Check Your Understanding p. 101 REVIEW Chapter 5 Review p. 102 #s 1 – 12 Heat Transfer Crossword Chapter 6: Controlling Heat Transfer Introduction Fire-walkers amaze tourists. They aim to convince their audiences that they have some sort of special abilities. But! Fire-walkers have learned to reduce the transfer of thermal energy from the red-hot coals to their feet. But how is this done? Introduction *Hint: Cooks test to see if a skillet is hot enough by dropping water into it. The pan is hot enough if the water drops dance across the surface of the skillet. Introduction We will re-visit the secrets of fire-walking later. We control heat transfer very day. Knowledge of differences in heat absorption is used in many ways. 6.1 Absorbing and Losing Heat Recall from Chapter 5: Water moderates the temperature of the land around it. In this chapter we will discuss another reason why that happens. Different materials absorb heat at different rates. This is referred to as heat absorption. 6.1 Absorbing and Losing Heat Specific Heat Capacity Different substances require different amounts of thermal energy to raise their temperature the same amount. This is true for all substances. Each substance requires a unique amount of heat gain or loss to change its temperature. 6.1 Absorbing and Losing Heat Specific heat capacity – measures a substance’s ability to absorb or lose heat Measured in joules per gram degrees Celsius 6.1 Absorbing and Losing Heat The specific heat capacity of a substance can change depending on its state. For example, solid water (ice) has a lower specific heat capacity than liquid water. This has to do in part with the relative attraction between the molecules. Molecules that are close together, as in a solid, have a stronger attraction to each other. They require more energy to get moving. The joule is named after James Prescott Joule (1818– 1899), a brewer and physicist. It is represented by the symbol J. 6.1 Absorbing and Losing Heat Example The specific heat capacity for water is What does this mean? One gram of water absorbs 4.19 joules of heat to raise its temperature 1°C One gram of water loses 4.19 joules of heat to lower its temperature 1°C Regardless of the amount of water, the amount of heat needed to change the temperature does not change. 6.1 Absorbing and Losing Heat Another example: The specific heat capacity of sand is This means: To increase the temperature of 1 gram of sand by 1 °C would require 0.66 J of energy. 6.1 Absorbing and Losing Heat Consider: SHC of water = SHC of sand = Which has a higher specific heat capacity? 6.1 Absorbing and Losing Heat Water! This means that it takes more energy to increase the temperature of water than it does to increase the temperature of sand. This is why sand on a sunny beach is much warmer than the shallow water nearby. 6.1 Absorbing and Losing Heat Substance Specific Heat Capacity Water 4.19 Motor oil 2.00 Vegetable oil 1.97 Air 0.995 glass 0.84 sand 0.66 Iron 0.45 copper 0.38 6.1 Absorbing and Losing Heat Warming Up and Cooling Down with Oceans Recall: oceans moderate shore areas Also, water has a high specific heat capacity. Oceans store more heat energy or thermal energy than you might expect. 6.1 Absorbing and Losing Heat With water’s large SHC, water can absorb, store or release much more thermal energy than land. That is another reason for land heating and cooling quicker than lakes and oceans. SHC also affects climate in other ways: On a hot day, water will absorb heat. This slows down the rise of temperature in the surrounding area. At night, water will release heat. This slows cooling in the surrounding area. 6.1 Absorbing and Losing Heat The secrets of fire-walking Water has a very high specific heat capacity (4.184 kJ/K kg), whereas coals have a very low one. Therefore the foot's temperature tends to change less than the coal's. Water also has a high thermal conductivity, and on top of that, the rich blood flow in the foot will carry away the heat and spread it. On the other hand, coal has a poor thermal conductivity, so the hotter body consists only of the parts of the coal which is close to the foot. 6.1 Absorbing and Losing Heat When the coal cools down, its temperature sinks below the flash point, so it stops burning, and no new heat is generated. Firewalkers do not spend very much time on the coals, and they keep moving. Calluses on the feet may offer an additional level of protection, even if only from pain; however, most people do not have calluses that would make any significant difference. 6.1 Absorbing and Losing Heat Practice! Check Your Understanding p. 110 6.2 Keeping Heat at Home Recall: heat moves from hot toward cold. This happens especially in the winter. Heat often leaks through windows, doors, and roofs. Proper insulation can help this problem. Insulation slows heat transfer. 6.2 Keeping Heat at Home With energy costs rising, Canadians want to make sure heat stays inside the house in winter. For Canadians, 50 to70 percent of energy costs go toward heating or cooling our homes. If insulation is inadequate, much energy is wasted. This is bad for the environment and for the home budget. 6.2 Keeping Heat at Home With insulation you get two benefits for the price of one. The same insulation that keeps heat in during the winter keeps heat out on a hot summer day. Knowledge of heat transfer teaches you how to keep your home warm in the winter and cool in the summer. But what makes a good insulator? 6.2 Keeping Heat at Home Insulation reduces heat transfer. A good insulator is the opposite of a good conductor. With good insulation, the three forms of heat transfer are slowed. 6.2 Keeping Heat at Home Heat convection and heat conduction can be minimized in two ways: First, create a partial vacuum between the areas to be insulated. This is done in vacuum bottles and in some double-glazed windows with an inert gas between the panes. Second, use trapped air. Still air provides 15 000 times better insulation than metal. 6.2 Keeping Heat at Home Substances used for insulation are chosen for their low thermal conductivity and their ability to trap air. Good conductors include cork, felt, cotton batting, magnesium carbonate, and spun glass or fibreglass. Asbestos is an excellent insulator but it is a health hazard and no longer used in construction. 6.2 Keeping Heat at Home Insulators work best when the air is dry. Moist air acts as a much better heat conductor than dry air. To keep insulation dry, fibreglass is covered with plastic sheeting before drywall is installed. Without the plastic sheet, moisture from inside the house — from cooking, bathing, and breathing — would infiltrate the insulation and reduce its effectiveness. Radiant heat loss can be lessened by installing aluminum foil, semi-reflective windows, and metal roofing. 6.2 Keeping Heat at Home R-value Air transfers heat when it is moved by convection currents. Air is an excellent insulator when it is held still. R-value is a measure of how well an insulating material slows heat transfer. Materials with high R-values are better insulators than those with low R-values. i.e.) R-12 loses heat faster than one with R-16 6.2 Keeping Heat at Home When materials are used together, the total R-value is the sum of the R-values of each material used. Example Walls in your home have 25 mm of expanded polystyrene and 25 mm of rigid urethane foam. What is the R-value of the insulation? 3.96 + 7.50 = R 11.46 Thickness of insulating material Approximate R-Value 25 mm of air space in a wall cavity 2.04 25 mm of air space with reflective surface on inside of wall cavity 5.54 25 mm of expanded polystyrene 3.96 25 mm of rigid urethane foam 7.50 25 mm of fibreglass 4.25 25 mm of solid wood 1.25 25 mm of wood shavings 2.42 25 mm of clay brick 0.11 25 mm of concrete 0.19 One thickness of glass 1.00 Thermal glass (2 thicknesses with air space) 1.80 6.2 Keeping Heat at Home Other Ways to Keep the Heat in Your House The empty space between the inside and outside wall of a house is called a wall cavity. Filling the cavity with insulation stops convection currents. 6.2 Keeping Heat at Home When insulation fills a wall cavity, there are many pickets of trapped, still air. Foam pellets and poured insulation work in this way. 6.2 Keeping Heat at Home Windows and Doors That Keep Heat In Windows and doors are two weak points when you try to keep a house warm. A single pane of glass is a poor insulator. Heat escapes quickly through glass. Leaks also develop around the panes and around the edge of the windows. 6.2 Keeping Heat at Home Older houses use double windows and doors – called storm windows and storm doors – to keep heat in. Today’s exterior doors and windows use double glazing. This provides a space of still air. Insulation value of this still air in improved when air is mixed with a gas such as argon. 6.2 Keeping Heat at Home Exterior doors used to be solid wood. Modern doors are cavities filled with insulation. Some are metal covered. To prevent heat transfer, there is a break in the metal between the inside and outside. 6.2 Keeping Heat at Home Did You Know? Even the smallest crack (1.5 mm) around the outside of one window, your furnace may burn an extra litre of fuel per day! 6.2 Keeping Heat at Home Controlling Heat Transfer Pizza parlours keep pizza warm by transporting it in insulated containers. As well as limiting heat transfer, the envelope must be washable. This limitation prevents the use of some materials. 6.2 Keeping Heat at Home A vacuum bottle uses several of the same technologies that keep your house warm in order to keep food and beverages warm. Inside is a double glass jar. One jar is fitted inside the other – similar to windows with a double pane. Some air is removed from between the two jars. That is where the name comes from. The space between is a partial vacuum. 6.2 Keeping Heat at Home Rubber or plastic keeps the glass away from the outer case. The cap is insulated. 6.2 Keeping Heat at Home Kitchen and Workshop Large appliances (i.e. Stoves, refrigerators, freezers, even dishwashers) are all insulated. Insulation slows heat transfer, keeping ovens hot and freezers cold. What makes a good insulator? Non-metals (i.e. Wood and plastic) 6.2 Keeping Heat at Home Examples: Plastic and/or wooden handles on kitchen utensils or pots and pans Aprons and oven mitts 6.2 Keeping Heat at Home Practice! Check Your Understanding p. 119 6.2 Keeping Heat at Home Lab Prep p. 118 When You’re Hot Challenge: Construct a container that will keep water hot for an hour. Design Criteria: Use any size container you want. Use any insulation materials you want. No thermoses, coolers, etc… 6.3 Keeping Yourself Warm The same techniques that keep our houses warm, keeps our bodies warm. For example, on a cold day we may choose to wear several layers. We choose inner layers for their open weave and thickness. Air trapped in the material serves as insulation. A windproof outer layer keeps warm air from escaping. 6.3 Keeping Yourself Warm Clothing insulates by holding air between fibres. The better clothes trap air, the better insulators they are. This is why thicker clothing tends to be warmer than thinner. Thicker clothing reduces air flow and maintains still air. Windproof material reduces or eliminates airflow through the fibres. 6.3 Keeping Yourself Warm Some of the warmest winter clothing contain down. Birds grow fluffy, down feathers. These feathers keep birds warm. Down is often quilted between the outer shell and inner lining of a vest or jacket. When down is fluffed, it holds air in place. The jacket material keeps air from blowing through the down and changing cold air for warm. 6.3 Keeping Yourself Warm Recall: a dry body is a warm body. Vigorous activity causes sweat. Water causes cooling as it evaporates. When working or playing outside, you want to protect yourself from getting and staying damp. When you start to sweat, remove one layer, possibly another. This allows heat to transfer out and your body to stay at the right temperature. When you stop moving you can replace those layers. 6.3 Keeping Yourself Warm On a cold day, up to 40 percent of body heat is lost through the head. Covering the head is important for heat retention. When a person becomes overheated in winter, a hat is often the first piece of clothing removed to cool the body and reduce perspiration. 6.3 Keeping Yourself Warm People of the North Inuit designed clothing is the warmest. Traditionally, Caribou Inuit clothes have two complete suits on cold days. The inner set is worn with fur next to the body. Body moisture is transferred through the fur and through the leather skin. 6.3 Keeping Yourself Warm Caribou fur is dense; individual hairs are hollow. Air trapped between and inside the hair provides insulation. The outer parka is worn with the fur outside – this is important for very cold days. The parka hood traps air in front of the wearer’s face. Frigid air is warmed before being breathed. This protects the wearer’s lungs. 6.3 Keeping Yourself Warm During extremely cold weather, water vapour from the lungs can condense and freeze on people’s faces and clothing. The hoods are designed to prevent condensation. The edge of the hood, where ice might form, is trimmed with fur. Ice does not stick to wolverine, wolf, or some dog fur. 6.3 Keeping Yourself Warm Caribou and Copper Inuit wear up to four layers on their feet in winter. Seal skin is preferred to boots because it is waterproof. Inuit parkas are much larger than we might expect. The large size allows wearers to bring their arms inside this warm space. 6.3 Keeping Yourself Warm Did You Know? Some motorcyclists have clothing with built in heaters! Heating elements are sewn into the clothing and warm areas of the body such as the torso where a lot of heat can be lost. The clothing runs off of a motorcycle battery and uses less power than a headlight. The amount of heat can be controlled using a palmsized computer. 6.3 Keeping Yourself Warm Did You Know? People outside in winter protect themselves from frostbite. Frostbite usually freezes hands, feet and the face. One way to warm a cold hand is to tuck it into your armpit for a few minutes! 6.3 Keeping Yourself Warm Keeping Cool Ever notice that people who live in warmer climate areas where more clothes? This is because they want to minimize heat transfer. They wear long, thick robes to protect the body from the Sun’s rays. These clothes are usually light in colour to help reflect heat and to allow body heat to escape. 6.3 Keeping Yourself Warm Oven mitts work in a similar manner. Their quilted material reduces heat transfer. Some include reflective material that also reduces heat transfer. 6.3 Keeping Yourself Warm Dressing for Intense Heat – or Cold Firefighters and deep-sea divers have to deal with major changes in temperature. But how? 6.3 Keeping Yourself Warm Firefighters’ suits are made of a special material. Many contain flame retardant chemicals. When flames or sparks come into contact with the suit, the fabric chars but does not burn. The charred material produces a layer of insulation that protects the firefighter from too much heat. 6.3 Keeping Yourself Warm Firefighters can suffer from heat stroke if their body temperature increases too much. Material on the inside of their fire suit absorbs body moisture. This helps to keep them cool. Firefighters must monitor their own bodies. If they get too hot, they could suffer from heatstroke – even if it’s in the winter! 6.3 Keeping Yourself Warm The fabrics used in firefighters’ suits are tested to determine their fire resistant qualities. They are tested by Scientists to see if they are safe enough for firefighters to wear. This is done by dressing a mannequin in fire gear and setting it on fire. These mannequins are equipped with sensors to measure the rate and amount of heat transfer from the fire. 6.3 Keeping Yourself Warm Even in hot climates, temperatures deep underwater can be as cold as winter. As a result, a diver’s suit should fit snugly. Tight diving suits prevent cold water next to the skin from causing cooling by conduction. If cold ocean water moved in and out of the suit, a diver would soon be cold. Water could pick up heat from the diver’s body and carry it off into the ocean. 6.3 Keeping Yourself Warm Dive suits are made of neoprene that has bubbles of nitrogen trapped in the fabric. The more gas trapped in the fabric, the higher the thermal value. These neoprene suits are well insulated to keep body heat inside. 6.3 Keeping Yourself Warm Dive suits also have hoods for the same reason that winter parkas have hoods. Underwater, a great amount of heat can be lost from a diver’s head. Some dive suits have titanium added to the side of the fabric that is next to the skin. The shiny titanium reflects heat back into the body. 6.3 Keeping Yourself Warm Practice! Check Your Understanding p. 125