* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atomic Theory Review
Low-energy electron diffraction wikipedia , lookup
Metallic bonding wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
History of chemistry wikipedia , lookup
Condensed matter physics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Atomic orbital wikipedia , lookup
Metalloprotein wikipedia , lookup
Chemical bond wikipedia , lookup
Double-slit experiment wikipedia , lookup
Electron scattering wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Geiger–Marsden experiment wikipedia , lookup
Elementary particle wikipedia , lookup
Electron configuration wikipedia , lookup
History of molecular theory wikipedia , lookup
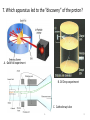
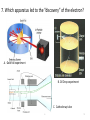
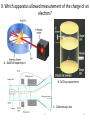
Atomic Theory Review A. 1 1. Which is associated with J.J. Thomson? A. Plum Pudding Model B. Planetary Model C. Solid Sphere Model D. Famous atomic experiment E. Electron Cloud Model A. 2 2. Which image is most like ideas associated with Dalton? A. Plum Pudding Model B. Planetary Model C. Solid Sphere Model D. Famous atomic experiment E. Electron Cloud Model c. 3 3. Which image is related to the discovery of the nucleus? A. Plum Pudding Model B. Planetary Model C. Solid Sphere Model D. Famous atomic experiment E. Electron Cloud Model d. 4 4. Which image best captures the orbital idea proposed by Bohr? A. Plum Pudding Model B. Planetary Model C. Solid Sphere Model D. Famous atomic experiment E. Electron Cloud Model b. 5 5. Which image is related most to today’s atomic theory? A. Plum Pudding Model B. Planetary Model C. Solid Sphere Model D. Famous atomic experiment E. Electron Cloud Model e. 6 6. Which image is most related to the work of Rutherford? A. Plum Pudding Model B. Planetary Model C. Solid Sphere Model D. Famous atomic experiment E. Electron Cloud Model d. 7 7. Which apparatus led to the “discovery” of the proton? A. Gold foil experiment B. Oil Drop experiment C. Cathode ray tube A. 8 7. Which apparatus led to the “discovery” of the electron? A. Gold foil experiment B. Oil Drop experiment C. Cathode ray tube c. 9 9. Which apparatus allowed measurement of the charge of an electron? A. Gold foil experiment B. Oil Drop experiment C. Cathode ray tube b 10 Which of the following is not part of Dalton’s Atomic Theory? A. All matter is composed of extremely small particles called atoms. B. Atoms of a given element are identical in size, mass and other properties. C. Atoms cannot be divided, created or destroyed. D. Atoms contain tiny particles called electrons. E. In chemical reactions, atoms are combined, separated or rearranged. d. 11 Read the postulates below. Which of Dalton’s assumptions is missing? 1. 2. 3. 4. A. B. C. Atoms of a given element are identical in size, mass and other properties. Atoms cannot be divided, created or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated or rearranged. Atoms may be split through fusion. The nucleus of the atom is held together by strong nuclear forces. Matter is composed of small particles called atoms. c 12 Complete the following items related to subatomic particles. 1. Name the three largest subatomic particles, identify their charges and state where they are found in the atom. 2. Which particles are in the nucleus? 3. Most of the atom’s volume is ___________. 4. Most of the atom’s mass is ___________. 1. Protons,+ , nucleus; neutrons, no charge, nucleus; electrons, -, in the cloud; 2. protons and neutrons; 3 . Empty space or the electron cloud; 4. in the nucleus Ionic compound charges 1. Which is a positive ion: A cation or an anion? 2. What is the charge of zinc in Zn3(PO4)2? 3. What is the charge on the iron atom in FePO4? 4. What is the name of FePO4? 5. What is the name of FeP? 6. Which of the following is incorrect? a) Sulfate is SO32- b) nitrate is an anion c) sodium chlorite is NaClO d) ammonium is a cation e) the nitride ion is N3- f) the oxide ion is a cation 1.cation; 2. +2, 3. +3; 4. iron(III)phosphate; 5. iron(III) phosphide 6. a,c,f 14 Practice naming and writing formulae • Use your worksheets or the “Quizlet” links to practice naming compounds and the polyatomic ions. A. 15