* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry of Life - juan-roldan
Stoichiometry wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Coordination complex wikipedia , lookup
Molecular orbital wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Biochemistry wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Chemical reaction wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Chemical element wikipedia , lookup
Periodic table wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Bent's rule wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Electrochemistry wikipedia , lookup
Water splitting wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Hydrogen bond wikipedia , lookup
Metalloprotein wikipedia , lookup
Electronegativity wikipedia , lookup
History of chemistry wikipedia , lookup
Bond valence method wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Molecular dynamics wikipedia , lookup
Extended periodic table wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Hydrogen atom wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Electrolysis of water wikipedia , lookup
Metallic bonding wikipedia , lookup
Atomic nucleus wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Electron configuration wikipedia , lookup
History of molecular theory wikipedia , lookup
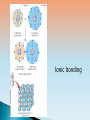
A knowledge of chemistry is essential for understanding organisms Important to biology are inorganic compounds, including water, simple acids and bases, and simple salts Elements ◦Substances that cannot be broken down into simpler substances by ordinary chemical reactions ◦Each has a chemical symbol Four elements comprise the mass of most organisms ◦Oxygen, carbon, hydrogen, and nitrogen In addition, other elements, such as calcium, and trace elements are present Functions of elements Atom ◦ The smallest portion of an element that retains its chemical properties Subatomic particles include ◦ Electron—carries a negative charge ◦ Proton—carries a positive charge ◦ Neutron—uncharged particle Every element has ◦A fixed number of protons in the atomic nucleus, known as the atomic number The periodic table is a chart of the elements arranged by atomic number The atomic mass of an atom ◦ Is a number that indicates how much matter it contains. ◦ Is expressed by the atomic mass unit (amu), also known as the dalton. ◦ The atomic mass= number of protons + number of neutrons Characteristics of protons, neutrons and electrons Isotopes ◦Are two or more forms of atoms of the same element ◦Contain the same number of protons and electrons, but the number of neutrons varies Radioisotopes break down and emit radiation Carbon Isotopes Electrons move through orbitals Electrons at the same principal energy level make up an electron shell Electrons in a shell distant from the nucleus have greater energy and are called Valence Electrons Valence electrons occupy the valence shell (outermost shell) Changes in electron energy levels are important in energy conversions in organisms Atomic orbitals The chemical behavior of an atom is determined by the number and arrangement of its valence electrons When the valence shell is not full, the atom tends to lose, gain, or share electrons A chemical compound consists of atoms of two or more elements Atoms combine in a fixed ratio Atoms may join to form a molecule A chemical formula describes the chemical composition of a substance ◦ Simplest formula ◦ Molecular formula ◦ Structural formula Molecular Mass ◦ Sum of the atomic masses of the component atoms of a single molecule Chemical reactions in an organism: ◦ Described by chemical equations ◦ Reactants are written on the left & products are written on the right ◦ Reactions can proceed simultaneously in both directions ◦ At dynamic equilibrium, forward and reverse rates of reaction are equal Chemical bonds ◦ Forces of attraction that hold atoms of a compound together ◦ The two principal types are Covalent bonds Ionic bonds Bond Energy ◦ Energy necessary to break a chemical bond Covalent bonds Covalent compound ◦ Share electrons between atoms ◦ Each atom has a filled valence shell ◦ Compound consisting mainly of covalent bonds ◦ Example is hydrogen gas molecule ◦ Bond can be single, double, or triple Covalent bonds Number of Covalent bonds Covalent bonds can be nonpolar or polar Ion ◦ Particle with one or more units of electrical charge ◦ Results when an atom gains or loses electrons Cations—positively charged ions (Na+) Anions—negatively charged ions ( Cl- ) ◦ Cations and anions are involved in biological processes, such as muscle contraction Sodium, potassium, and chloride ions are essential for this nerve cell to stimulate these muscle fibers Ioninc bonds ◦ Formed due to attraction between a cation and an anion ◦ An example of ionic bond is the attraction between sodium ions and chloride ions Ionic bonding Hydrogen Bonds ◦ Form between an atom with partial negative charge and a hydrogen atom covalently bonded to oxygen or nitrogen ◦ Readily formed and broken ◦ While individually weak, hydrogen bonds are strong when present in large numbers Hydrogen bonding Many energy conversions in a cell involve an electron transfer from one substance to another Known as oxidation-reduction, or redox reaction Water Water is Polar (due to its uneven distribution of charges) Large part of the mass of most organisms is water Water is important as internal constituent and environmental factor Water facilitates chemical reactions: ◦ Hydrophilic substances—interact readily with water, such as table salt ◦ Hydrophobic substances—not disrupted or dissolved by water, such as fats Water exists as gas, liquid, or solid Hydrogen bonds are formed or broken as water changes state Acid ◦ Substance that dissociates in solution to yield hydrogen ions and an anion Base ◦ Substance that dissociates to yield a hydroxide ion and a cation when dissolved in water The degree of a solution’s acidity is expressed in pH Definition of pH ◦ Measure of how acidic or basic a substance is ◦ The negative logarithm of the hydrogen ion concentration ◦ Expressed in moles per liter Neutral solution Acidic solution Basic solution An acid and a base react to form a salt plus water A buffer is a substance that resists pH changes in a solution. Buffers a important substances in biological systems. ◦ pH of 7 ◦ pH value of less than 7 ◦ pH greater than 7 pH values of common solutions