* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Slide 1 - Statnet
Bioterrorism wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Influenza A virus wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Oesophagostomum wikipedia , lookup
Trichinosis wikipedia , lookup
2015–16 Zika virus epidemic wikipedia , lookup
Leptospirosis wikipedia , lookup
Hepatitis C wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Orthohantavirus wikipedia , lookup
Hepatitis B wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
West Nile fever wikipedia , lookup
West African Ebola virus epidemic wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
Henipavirus wikipedia , lookup
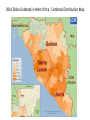
Network Modeling Meeting: Ebola NIH Highlights •Ebola virus disease (EVD), formerly known as Ebola haemorrhagic fever, is a severe, often fatal illness in humans. •The virus is transmitted to people from wild animals and spreads in the human population through human-to-human transmission. •The average EVD case fatality rate is around 50%. Case fatality rates have varied from 25% to 90% in past outbreaks. •The first EVD outbreaks occurred in remote villages in Central Africa, near tropical rainforests, but the most recent outbreak in west Africa has involved major urban as well as rural areas. •Community engagement is key to successfully controlling outbreaks. Good outbreak control relies on applying a package of interventions, namely case management, surveillance and contact tracing, a good laboratory service, safe burials and social mobilisation. •Early supportive care with rehydration, symptomatic treatment improves survival. There is as yet no licensed treatment proven to neutralise the virus but a range of blood, immunological and drug therapies are under development. •There are currently no licensed Ebola vaccines but 2 potential candidates are undergoing evaluation. A Brief History: • The first known outbreak of EVD occurring between June and November 1976 in Nzara, South Sudan, and was caused by Sudan virus (SUDV). The Sudan outbreak infected 284 people and killed 151. • On 26 August 1976, a second outbreak of EVD began in Yambuku, DRC. This outbreak was caused by EBOV, formerly designated Zaire ebolavirus, which is a different member of the genus Ebolavirus than in the first Sudan outbreak. • EVD in humans is caused by four of five viruses of the genus Ebolavirus. The four are Bundibugyo virus (BDBV), Sudan virus (SUDV), Tai Forest virus (TAFV) and one simply called Ebola virus (EBOV, formerly Zaire Ebola virus). EBOV, species Zaire ebolavirus, is the most dangerous of the known EVD-causing viruses, and is responsible for the largest number of outbreaks. The fifth virus, Reston virus (RESTV), is not thought to cause disease in humans. • The current outbreak in west Africa, (first cases notified in March 2014), is the largest and most complex Ebola outbreak since the Ebola virus was first discovered in 1976. There have been more cases and deaths in this outbreak than all others combined. It has also spread between countries starting in Guinea then spreading across land borders to Sierra Leone and Liberia, by air to Nigeria and the United States, and by land to Senegal. • As of November 11, 2014 (Updated November 14, 2014) Total Cases: 14413 Laboratory-Confirmed Cases: 8920 Total Deaths: 5177 • • • 2014 Ebola Outbreak in West Africa - Outbreak Distribution Map Transmission • Initial patient becomes infected through a spillover event - contact with an infected animal, such as a fruit bat or primate (apes and monkeys) • Between people, Ebola disease spreads only by direct contact with the blood or body fluids of a person who has developed symptoms of the disease. – saliva, mucus, vomit, feces, sweat, tears, breast milk, urine and semen • • – – – – – – • • Only people who are very sick are able to spread Ebola disease in saliva whole virus has not been reported to be transmitted through sweat. Most people spread the virus through blood, feces and vomit. Contact with surfaces or objects contaminated by the virus, particularly needles and syringes, may also transmit the infection. The virus is able to survive on objects for a few hours in a dried state and can survive for a few days within body fluids. The Ebola virus may be able to persist for up to 7 weeks in the semen of survivors after they recovered Ebola may also occur in the breast milk of women after recovery, and it is not known when it is safe to breastfeed again Entry points for the virus include the nose, mouth, eyes, open wounds, cuts and abrasions. 60% of the cases of Ebola infections in Guinea during the 2014 outbreak are believed to have been contracted via unprotected (or unsuitably protected) contact with infected corpses during certain Guinean burial rituals Health-care workers / family members treating those who are infected are at greatest risk of getting infected themselves. Infection • Symptoms may appear anywhere from 2 to 21 days after exposure to Ebola, but the average is 8 to 10 days. • First symptoms are the sudden onset of fever fatigue, muscle pain, headache and sore throat. This is followed by vomiting, diarrhoea, rash, symptoms of impaired kidney and liver function, and in some cases, both internal and external bleeding (e.g. oozing from the gums, blood in the stools). • People who recover from Ebola infection develop antibodies that last for at least 10 years. CDC's Model for West Africa Ebola Outbreak Summarized by Li Wang, 11/14 SIIR compartmental model • Deterministic model with 4 compartments (susceptible, incubation, infectious, recovery) • Time step = 1 day. Model period is from 2/14 to 12/14. • Model population is homogenous (no distinction of sex, age, location, etc.), of entire country (10 mil). • Model parameters are chosen by inspection to fit data through 6/18/14. Disease model • New cases go into Incubation compartment • Can come from transmission or imported; initially set to 25 • Length of incubation follow log-normal distribution (max 25 days) Incubationi Distribution 0.180 0.160 0.140 0.120 0.100 0.080 0.060 0.040 0.020 0.000 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Days Disease model • After incubation period, case moves to Infectious compartment • Remain infectious for 6 days, including burial period. Has a large effect on epidemic. • Then the case moves to Recovery compartment. This covers both recovery and death. Transmission model • Infectious cases are assigned to 3 categories: Hospitalized, Home isolation, or No isolation • The proportion of cases in each category changes to reflect public health response to the epidemic 1.00 % Patients by category over time 0.90 0.80 % Patients 0.70 0.60 0.50 0.40 0.30 0.20 0.10 0.00 Days Hospitalization Eff Home Isolation* No Isolation Transmission • The daily transmission risk is the probability of a person to transmit, per day. This is done deterministically, so if the risk is 0.3, then the number of new cases = 0.3 x number of infectious. • Different by category: – Hospitalized: 0.02 – Home isolation: 0.03 – Non isolated: 0.30 • Importance of public health response! Effective isolation will stop the epidemic. Assumptions and limits • The model parameters are chosen to fit data from the first 135 days. • Under-reporting: the corrected model assumes that the number of actual cases are 2.5 times the reported. • CDC's deterministic model does not provide any measure of uncertainty. The WHO report does. • "The mean time from the onset of symptoms to hospitalization, a measure of the period of infectiousness in the community, was 5.0±4.7 days ... The mean time to death after admission to the hospital was 4.2±6.4 days, and the mean time to discharge was 11.8±6.1 days." - WHO Projection Daily clinical cases of Ebola 350 Ebola cases per day: Estimates based on data from Generic, uncorrected and corrected for potential under -reporting 300 250 200 Daily cases (corrected) 150 Daily cases (uncorrected) Total: - - 19,957 —51,962 100 50 0 Days 1.00 % Patients by category over time % Patients 0.80 0.60 0.40 0.20 0.00 Hospitalization Days Eff Home Isolation* No Isolation Daily clinical cases of Ebola Projection - Delayed response 1,800 1,600 1,400 1,200 1,000 800 600 400 200 0 Ebola cases per day: Estimates based on data from Generic, uncorrected and corrected for potential under -reporting Daily cases (corrected) Total: - - 72,754 —190,717 Daily cases (uncorrected) Days 1.00 % Patients by category over time % Patients 0.80 0.60 0.40 0.20 0.00 Hospitalization Days Eff Home Isolation* No Isolation Model improvements • What more can be done with available data? (location of cases, size of communities) • Model the delay between infectious symptoms and hospitalization or isolation • Simulate the transmission process to get a measure of uncertainty. References • http://www.cdc.gov/mmwr/preview/mmwrhtml/su6303a1.htm • EbolaResponse model spreadsheet: http://dx.doi.org/10.15620/cdc.24900 • WHO article: http://www.nejm.org/doi/full/10.1056/NEJMoa1411100?query=featured_ home&&#t=article